Abstract
BACKGROUND AND PURPOSE: Brain MR imaging is recommended in children with cerebral palsy. Descriptions of MR imaging findings lack uniformity, due to the absence of a validated quantitative approach. We developed a quantitative scoring method for brain injury based on anatomic MR imaging and examined the reliability and validity in correlation to motor function in children with hemiplegia.
MATERIALS AND METHODS: Twenty-seven children with hemiplegia underwent MR imaging (T1, T2-weighted sequences, DTI) and motor assessment (Manual Ability Classification System, Gross Motor Functional Classification System, Assisting Hand Assessment, Jebsen Taylor Test of Hand Function, and Children's Hand Experience Questionnaire). A scoring system devised in our center was applied to all scans. Radiologic score covered 4 domains: number of affected lobes, volume and type of white matter injury, extent of gray matter damage, and major white matter tract injury. Inter- and intrarater reliability was evaluated and the relationship between radiologic score and motor assessments determined.
RESULTS: Mean total radiologic score was 11.3 ± 4.5 (range 4–18). Good inter- (ρ = 0.909, P < .001) and intrarater (ρ = 0.926, P = < .001) reliability was demonstrated. Radiologic score correlated significantly with manual ability classification systems (ρ = 0.708, P < .001), and with motor assessments (assisting hand assessment [ρ = −0.753, P < .001]; Jebsen Taylor test of hand function [ρ = 0. 766, P < .001]; children's hand experience questionnaire [ρ = −0. 716, P < .001]), as well as with DTI parameters.
CONCLUSIONS: We present a novel MR imaging–based scoring system that demonstrated high inter- and intrarater reliability and significant associations with manual ability classification systems and motor evaluations. This score provides a standardized radiologic assessment of brain injury extent in hemiplegic patients with predominantly unilateral injury, allowing comparison between groups, and providing an additional tool for counseling families.
ABBREVIATIONS:
- CP
- cerebral palsy
- GMFCS
- Gross Motor Functional Classification System
- MACS
- Manual Ability Classification System
- AHA
- Assisting Hand Assessment
- JTTHF
- Jebsen Taylor Test of Hand Function
- CHEQ
- Children's Hand Experience Questionnaire
The role of neuroimaging in assessing patterns of injury in patients with cerebral palsy (CP) is well established. MR imaging of the brain for evaluation of children with CP is a practice recommended by the American Academy of Neurology since 2004.1 Major reviews of the literature support this practice guideline.2⇓–4 Korzeniewski et al2 concluded that neuroradiologic imaging modalities are making significant contributions to our understanding of CP. However, there are significant inconsistencies in descriptions of radiologic findings. In their systematic review of the literature, 42 studies relating to neuroimaging evaluation of patients with CP were assessed and were found to use more than 100 different terms to describe brain injury in these patients, making comparison between studies very difficult. In their review, Krägeloh-Mann and Horber3 highlighted the potential of MR imaging to elucidate etiology and pathogenesis in CP but found a lack of consensus on classification of MR imaging results. In their opinion, a standardized classification of MR imaging results would be helpful for data collection in CP registries. This was acknowledged by the Surveillance of Cerebral Palsy in Europe network, which developed a recommended standardized method for reporting brain MR imaging results.5,6 Arnfield et al4 investigated the relationship between the brain structure on MR imaging and motor outcomes in children with CP by reviewing the related literature. They found a relationship between the type of brain lesion as seen on MR imaging and 2 clinical outcomes: Gross Motor Functional Classification System (GMFCS)7,8 and type of CP. The difficulties they encountered in comparing MR imaging results between different studies led them to emphasize the importance of developing a validated quantitative approach to classification of brain injury on MR imaging.
In our medical center, we developed a quantitative scoring method for brain injury based on anatomic MR imaging of the brain. The purpose of this study was to examine the reliability and validity of the scoring system in correlation to motor function status in a cohort of children with hemiplegia.
Materials and Methods
Participants
The study group comprised 27 children with clinical signs of hemiplegia, recruited from the Regional Pediatric Neurology Unit at the Tel Aviv Sourasky Medical Center and Guy's and St Thomas' NHS Foundation Trust and affiliated Child Development Centres, as part of a study examining motor intervention in children with hemiplegia.9 Inclusion criteria were clinical signs of spastic hemiplegia as assessed by a neurologist, attendance at a mainstream educational institution, and independence of mobility. Exclusion criteria were intractable seizures, prior surgical intervention, and any contraindications to MR imaging.
This study was approved by the Institutional Review Board and National Research Ethics Committee of the respective hospitals, and fully informed consent was obtained from parents and/or children over 18 years of age.
Motor Function Assessment
Motor classification included rating according to the Manual Ability Classification System (MACS) and the GMFCS. The MACS classifies ability to handle objects in important daily activities across a 5-point scale; children at level I handle most objects easily and at level V are severely limited in their ability.10 The GMFCS is a measure of spontaneous functional mobility.7 Participants were also assessed with the Assisting Hand Assessment (AHA; version 4.3),11 the Jebsen Taylor Test of Hand Function (JTTHF),12 and the Children's Hand Experience Questionnaire (CHEQ).13 AHA tests spontaneous use and performance of the affected hand in functional/play-based tasks; higher scores represent better bimanual skills. JTTHF is a timed test of manual dexterity where higher scores represent poorer unimanual skills. CHEQ is a 29-item questionnaire of affected hand use in daily bimanual activities; higher scores represent better hand use.
MR Imaging
All participants underwent MR imaging and motor assessment on the same day, with training in a mock scanner before the actual scan. MR imaging scans were performed on 1 of 2 3T scanners (Signa Excite, Milwaukee, Wisconsin), the first at Tel Aviv Sourasky Medical Center, Israel (19 participants), and the second at the Institute of Psychiatry, London, United Kingdom (8 participants). The scanning protocol, which was matched between sites, included:
-
• 2D sagittal T1-weighted conventional spin-echo. TR = 400 ms, TE = 10 ms; 21 × 5 mm sections with a 1.5 mm gap; matrix size 256 × 160 over a 240 mm FOV.
-
• 2D axial T2-weighted fast spin echo. TR = 4000 ms, TE = 137 ms; 36 × 4 mm sections with no section gap; matrix size 384 × 224 over a 240 mm FOV.
-
• 2D axial FLAIR. TR = 9000 ms, TE = 144 ms, TI = 2100 ms; 32 × 4 mm sections with no section gap; matrix size 256 × 192 over a 240-mm FOV.
-
• Gradient-echo T2*. FOV/matrix = 240 mm2/512 × 512; TR/TE = 320/20 ms.
-
• Axial 3D high-resolution anatomic T1-weighted fast spoiled gradient-echo imaging. (FOV)/matrix = 240–256 mm2/256 × 256; TR/TE = 8.6/3.3 ms.
-
• DTI acquired along 19 diffusion gradient directions (b = 1000 s/mm2) and 1 with no applied diffusion gradient, (FOV/matrix = 220 mm2/acquired matrix of 128 × 128; reconstructed to 256 × 256; TR/TE = 11,000/91 ms).
Radiologic Scoring
A scoring system was devised, adding points for positive findings, as shown in the On-line Appendix. The score covered 7 aspects across 4 domains: 1) number of affected lobes, 2) volume and type of white matter injury, 3) extent of gray matter damage, and 4) major white matter tract injury. Scoring was dichotomous, with 1 point for pathology and 0 for normal structure, with the exception of white matter volume loss, which was graded as explained below. Total radiologic score was obtained by summing all points. Thus, a child with a completely normal scan would get a score of 0. Brain injury with unilateral hemispheric involvement may reach a maximum score of 18. In the presence of bilateral brain injury, each hemisphere was scored separately and summed to give the total score. The following aspects of brain injury were assessed:
-
1. Number of affected lobes: for each lobe affected, 1 point was scored, up to a maximum of 4 points if all 4 lobes in the injured hemisphere were affected.
-
2. White matter injury:
-
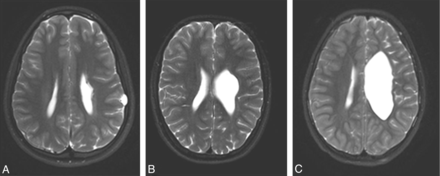
a. Volume loss of white matter in the affected regions was assessed in comparison with the unaffected side, by averaging a few measurements of white matter width at the region of abnormality and comparing them with the unaffected hemisphere. Extent of white matter volume loss was described as none (0 points), mild (1 point) if less than 40% decrease in volume, moderate (2 points) if approximately 40%–60% decrease in volume, and severe (3 points) for greater than 60% decrease in volume (Fig 1).
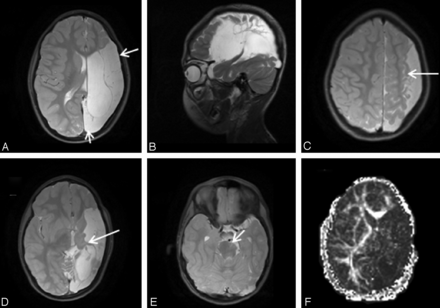
-
b. Presence of T2 signal changes reflecting gliosis, regardless of extent, was scored 1 point and absence of gliosis 0 points. (Fig 2A, -B).
-
c. Presence of parenchymal cystic changes (not including ex-vacuo dilation of the ventricle) was scored 1 point and absence of cystic changes 0 points. (Fig 2C).
-
-
3. Gray matter injury:
-
a. Cortical gray matter abnormality was scored 1 point, regardless of extent. Cortical gray matter abnormality was reflected by thinning and/or signal abnormality in case of injury (Fig 2A–C), and by thickening and abnormal structure in case of congenital malformation.
-
b. Deep gray matter injury was evaluated for extent of basal ganglia and thalami involvement by assigning 1 point for each to a maximal score of 4 points if caudate, putamen, globus palidus, and thalamus were affected (Fig 2C).
-
-
4. Major white matter tract injury was scored by assigning 1 point for presence of signal changes in each of the following: the internal capsule anterior limb, internal capsule posterior limb, and external capsule (Fig 2C). In addition, presence of cerebral peduncles asymmetry at the level of the midbrain was also scored 1 point (Fig 2D).
Application of the radiologic scoring method in 2 different children is presented in Figs 3 and 4.
Axial T2-weighted images at the level of the lateral ventricles from 3 different patients demonstrating different levels of white matter loss: A indicates mild; B, moderate; and C, severe.
A and B, Axial T2-weighted images demonstrate high T2 signal in the frontal subcortical white matter consistent with white matter gliosis. There is thinning and high T2 signal in the frontal cortex consistent with cortical gray matter injury. C, Axial T2-weighted image at the level of the basal ganglia of a different patient demonstrates cystic changes involving both cortical and deep gray matter, with extensive involvement of the basal ganglia, thalamus, and adjacent major white matter tracts. D, Axial T2-weighted image at the level of midbrain-pons junction demonstrating significant asymmetry of cerebral peduncles, reflecting left sided Wallerian degeneration.
A series of axial T2-weighted images from a 7-year-old boy with left hemiplegia and MACS score of 1, with prematurity-related brain injury (periventricular leukomalacia + focal infarct). A, Right-sided perirolandic encephalomalacia involving frontal and parietal lobes (2 points); white matter changes include gliosis (1 point), no cystic changes. B–D, Assessment of white matter volume loss: the width of white matter was measured in the affected area and compared with the contralateral side, calculating percentage of volume loss. At least 3 measurements were performed and averaged; in this case, the average was in the range of moderate volume loss (40%–60%) (2 points). E, Presence of cortical gray matter changes (1 point). F, No involvement of deep gray matter and major white matter tracts. The total score was 6.
Images from an 8-year-old boy with right hemiplegia and MACS score of 3, with a background of left MCA infarct, born at term. A, Axial T2-weighted image, and B, sagital T2-weighted image, both demonstrate involvement of all 4 lobes (4 points). Extensive cystic and gliotic changes are present (2 points). In this case there is obvious severe white matter volume loss, which makes actual measurement redundant (3 points). There is extensive loss of cortical gray matter with thinning and gliosis in adjacent gray matter (1 point) as shown in C. D, Extensive involvement of deep gray matter structures (4 points), as well as major white matter tracts including external capsule and anterior and posterior limbs of the internal capsule (3 points). E, Asymmetry of the pyramids (1 point). When DTI is available, the anisotropy map can help in delineating the white matter tract involvement as seen in F. The total score was 18.
Scoring was performed by a pediatric radiologist with 6 years' experience in pediatric neuroradiology. The scoring was performed a second time, with a period of at least 6 months between assessments, to evaluate intraobserver variability. An additional assessment was performed in all cases by a second radiologist at the beginning of her neuroradiology training to evaluate interobserver variability.
DTI Analysis
DTIStudio software (Johns Hopkins University, Baltimore, Maryland) was used for DTI analyses as per our published protocol.14 In brief, tractography analysis was performed to reconstruct the corpus callosum by using a streamline fiber-tracking method with the fiber assignment by continuous tracking algorithm.15 The Witelson parcellation scheme16 was used to segment the corpus callosum into 3 segments: genu, midbody, and splenium. Region of interest analysis was performed for the left and right posterior limbs of the internal capsule by using ROIEditor software (Johns Hopkins University, Baltimore, Maryland). Mean values of axial diffusivity, radial diffusivity, mean diffusivity, and fractional anisotropy were calculated for each fiber/region of interest.
Statistical Analysis
Spearman correlations were used to assess inter- and intrarater reliability of total radiologic score (continuous item), whereas Cohen κ was used to evaluate inter- and intrarater reliability of each individual item (dichotomous or ranked items), accounting for the effects of chance agreement.17 A score of at least 0.6 is taken as substantial agreement, whereas 0.8 and greater represents a very high level of agreement.18
ANOVA test and Scheffe post hoc tests were used to examine differences in radiologic score according to MACS and GMFCS classification. Spearman correlation was used to further assess this relationship. Spearman correlations were applied and scatterplots plotted to determine the relationship between radiologic score and motor assessments including the AHA, JTTHF, and CHEQ scores. Means and standard deviations were calculated for each fiber tract/region of interest. Pearson correlations were calculated between fractional anisotropy and diffusivity parameters of each fiber tract/region of interest and the total radiologic scores. Data were analyzed using SPSS version 17.0 (IBM, Armonk, New York).
Results
The study group comprised 27 children (15 male, 12 female), mean age 10.9 ± 3.2 years (range 7.0–18.7 years). Seven children (26%) were born prematurely (mean gestational age = 29 ± 3 weeks). Of the remaining term born children, 13 had perinatal brain injury, 3 presented with congenital malformation, 1 child had traumatic brain injury at 3 months of age, and 3 children had stroke at a later age, at 18 months, 2.5 years, and 7.5 years of age. Most subjects had right hemiplegia (70%; n = 19). GMFCS score ranged from 1 (40%) to 2 (60%) whereas MACS score ranged from 1 to 3, with a mean score of 2.0 ± 0.7. Mean total radiologic score (mean of 3 ratings from 2 radiologists) was 11.3 ± 4.5, with a range of 4–18 (On-line Table).
Correlation analyses showed good inter- (ρ = 0. 909, P < .001) and intrarater (ρ = 0. 926, P < .001) reliability for total radiologic score. When analyzed item-by-item, substantial agreement was found, with mean κ score 0.687 for intrarater, and 0.676 for interrater variability (Table 1).
Intra- and interrater reliability
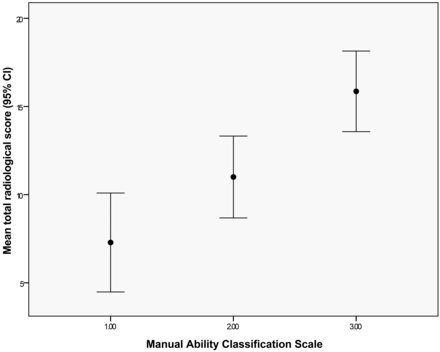
A strong positive correlation was evident between radiologic score and MACS severity (ρ = 0.708, P = < .0001). On further analysis, a 1-way ANOVA showed that mean radiologic score differed significantly between the 3 MACS classifications, with lower MACS score (representing less impairment) corresponding to lower radiologic score [F(2,24) = 11.571, P = .003] (representing less brain injury based on imaging). Post hoc tests showed a significant difference between MACS 1 and 3 according to radiologic score, differentiating the 2 groups (Fig 5). No significant correlation was detected between radiologic score and GMFCS (ρ = 0.281; P = .156).
Mean and 95% confidence intervals of radiologic scores according to MACS classification.
Strong, significant correlations were further detected between radiologic score and motor assessments, including the AHA (ρ = −0.753, P < .001), JTTHF (ρ = 0. 766, P < .001), and CHEQ (ρ = −0. 716, P < .001) (Fig 6). As radiologic score increased (representing increased brain injury), motor performance decreased, indicated by lower AHA and CHEQ scores and longer JTTHF reaction times.
Scatterplots showing correlation between radiologic score and motor assessments on the AHA, JTTHF, and CHEQ scores.
Total Radiologic Score and White Matter Integrity
Two children did not undergo the DTI scan (subjects 15 and 24) and 1 child (subject 21) was excluded due to movement artifacts. In 2 children (subjects 1 and 7), all segments of the corpus callosum and the affected posterior limbs of the internal capsule could not be reconstructed due to the large size of the lesion. In 1 child (subject 12), the midbody of the corpus callosum, and in another (subject 9), the affected posterior limbs of the internal capsule could not be reconstructed due to the lesion. These children were excluded, leaving 20 children whose DTI data could be analyzed.
Significant correlations were found between the white matter integrity of the corpus callosum and the total radiologic score, in the genu, midbody, and splenium. Higher diffusivity values, reflecting reduced white matter integrity, were associated with higher radiologic score (Table 2).
Correlations between DTI values in the corpus callosum and total radiologic score
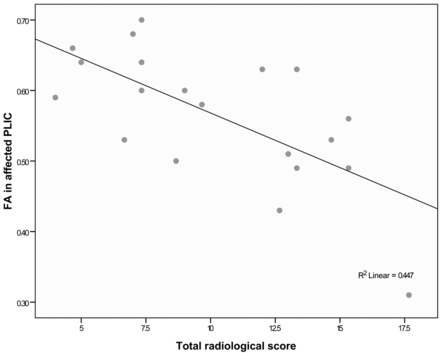
Significant correlations were also found between the white matter integrity of the affected posterior limbs of the internal capsule and the total radiologic score. The lower the fractional anisotropy in the affected posterior limbs of the internal capsule (indicating reduced white matter integrity), the higher the total radiologic score (r = −0.668, P < .001) (Fig 7). There were no significant correlations between the total radiologic score and the white matter integrity of the less affected posterior limbs of the internal capsule.
Scatterplot showing correlation between radiologic score and fractional anisotropy in the affected posterior limbs of the internal capsule.
Discussion
We present a novel scoring system, based on diagnostic MR imaging study of the brain, developed to describe the extent of brain injury in a quantitative fashion. Our results show high inter- and intrarater reliability and significant correlation with motor classification and function on motor assessments of hand function of children with hemiplegia.
In recent years, studies have suggested that the combination of type, location, and extent of brain lesion in patients with CP is a stronger predictor of hand function than lesion type alone.19,20 These studies highlight the importance of a standardized approach for assessing extent of brain damage. In this study, we report on a point value MR imaging–based scoring method to estimate extent of brain injury in children with hemiplegia and to determine its correlation to clinical status, independent of the underlying etiology.
Feys et al21 reported a systematic approach to describing brain injury in addition to pattern recognition; they used a checklist of anatomic structures involved in brain injury, assigning 1 point for pathology and 0 for normal structure. These data were used to investigate the association between each affected neuroanatomic structure to upper limb function. Their study highlighted the importance of lesion location to upper limb function; however, they did not assign a score profile per child, thus preventing comparison between patients.
A methodical scoring system for pediatric brain injury was reported by Woodward et al,22 who proposed a standardized scoring system of neonatal brain injury. The authors found significant associations between the qualitative measures of cerebral white matter and gray matter abnormalities on MR imaging at term-equivalent age and the subsequent risks of adverse neurodevelopmental outcomes at 2 years of age among very preterm infants. Additional studies reported qualitative and quantitative methods for assessing the extent of injury to the premature brain at term equivalent age.23⇓–25 Using these scoring systems in our population was not possible as they rely on the unique imaging features of the immature neonatal brain which do not apply to MR imaging studies performed on older children with mature brains.
In our scoring method, we aimed to simplify the brain MR imaging evaluation and minimize interrater variability by using dichotomous items; each brain injury characteristic was scored 1 for a positive finding and 0 if no pathology was noted. Only white matter volume loss was graded for extent with point values ranging from 0, if no volume loss was noted, to 3 for most severe volume loss, adding an element of qualitative evaluation. This method appears reliable and reproducible as reflected by very good intra- and interobserver correlation. The fact that the second rater had recently finished her residency and was at the beginning of her neuroradiology fellowship suggests that this method is simple and does not require extensive experience to use.
Furthermore, the system we developed is consistent with the classification of MR imaging recommended by the Surveillance of Cerebral Palsy in Europe network,6 but goes further in quantifying the extent of brain damage to enable comparison between children and across studies.
Correlation between DTI measurements and clinical status was previously reported by our group,14 though this is a larger cohort with additional children that were not included in the previous study. The strong correlation we found between total radiologic score and DTI measurements further supports the reliability of this method, as it seems the scoring based on anatomic imaging reflects well the microstructural changes that are measured in the DTI evaluation.
Several studies demonstrated significant correlation between deep gray matter involvement and worse upper limb motor function.19,21,26 Holmefur et al19 reported worse hand function to be associated with combined involvement of both basal ganglia and thalamus, independent of the basic type of brain lesion. Based on diffusion MR imaging studies, Holmström et al27 suggested significant changes in major white matter tracts also correlate with hand function. Our radiologic score took this into account by giving detailed scoring to each aspect of the deep gray matter and major white matter tracts rather than clumping these structures together for a single score. This embedded relative “weighting” of extent of deep gray matter involvement may explain the good correlation between the radiologic score and the functional status of patients.
Looking at the correlation between the radiologic scores and the MACS grades (Fig 5) it appears that the children with MACS 3 have a radiologic score above 11, and children with MACS 1 have a radiologic score below 13, which suggests good discrimination between MACS 1 and 3. Children with MACS 2 have more variable injury scores that overlap with 1 and 3. Although this assessment is limited by the lack of higher MACS grades in this group of patients, and by the small sample, these preliminary results do suggest possible prediction of MACS grade by radiologic score.
Our study did not find a correlation between the radiologic score and GMFCS. This is likely related to the relatively similar high function that children with hemiplegia have in regards to gross motor function, as measured by GMFCS scores of I and II only in our study group.
In this study group, children had predominantly unilateral abnormalities. Only 1 child had bilateral abnormalities (subject 27). The combined radiologic score from each hemisphere was used for the statistical analysis. Feys et al21 demonstrated a lack of impact of bilateral abnormalities on the use of the hemiplegic hand. For this specific child, with a high MACS rating, the radiologic score of the hemisphere responsible for the hemiplegic hand alone fell below the 95% CI range when correlating with MACS, suggesting a possible impact of bilateral abnormalities on the use of the hemiplegic hand. However, no conclusions can be made based on a single case. Other studies have described bilateral abnormalities in larger numbers of children with unilateral hemiplegia.19,21 The lack of significant bilateral abnormalities in our study group reflects the purposive sampling of children with clinical presentation of predominant unilateral impairment in view of the motor intervention protocol. In addition, the sample had a smaller percentage of patients with prematurity-related brain injury, compared with other studies, which tends to cause higher percentage of bilateral injury. Our results support further research to validate this scoring method in the presence of bilateral abnormalities in a greater number of children.
The main limitations of this study are the relatively small sample and the fact that all participants exhibited predominantly unilateral hemiplegia with relatively good gross motor functional status. It should be noted that most studies involving imaging/neurophysiology of children with brain injury are by nature small,28⇓⇓⇓–32 hence the need for a reliable scale that can be used to compare results. Although it remains to be seen whether the scoring method is effective in other types of motor disorders or with significant bilateral brain abnormalities, our results are promising and we believe justify further validation of the scoring method with a larger number of patients with variable subtypes of CP and acquired brain injury.
Conclusions
We present a novel MR imaging–based scoring system to describe brain injury in children with hemiplegia, which demonstrated high inter- and intrarater reliability and was significantly associated with MACS classification and motor evaluations. The significant correlations demonstrated between the proposed score and motor function status are a promising first step for the validation of this scoring system in children with hemiplegia. This scoring system may fill an important gap by providing a standardized radiologic assessment of brain injury extent in patients with predominantly unilateral brain injury and thus will allow comparison between registries or study groups. It may also provide the clinician an additional tool for counseling families regarding prognosis. Further studies will allow for better characterization of the properties of this instrument.
Acknowledgments
We thank the children and families who participated, the Guy's and St Thomas' Charity and Marnie Kimelman Trust for funding, and Geoff Charles-Edwards from the Biomedical Engineering Department and Gareth Barker of the Department of Neuroimaging, Institute of Psychiatry at Kings College London, for providing the data from the UK participants.
Footnotes
Disclosures: Dido Green—RELATED: Grant: Guy's and St Thomas' Charity,* Marnie Kimelman Trust,* Comments: These monies were paid directly to institute for project expenses of venue, equipment, and delivery of project. I received no salary payment or any funds for this project. *Money paid to institution.
References
- Received November 27, 2013.
- Accepted after revision January 30, 2014.
- © 2014 by American Journal of Neuroradiology