Abstract
BACKGROUND AND PURPOSE: Patients with vascular parkinsonism have higher cognitive decline and more basal ganglia lesions. We aimed to evaluate the relationship of cognitive impairment with functional connectivity between the basal ganglia and cingulate cortex in vascular parkinsonism.
MATERIALS AND METHODS: Thirty patients (8 with vascular parkinsonism and 22 with Parkinson disease) and 23 controls were enrolled. The Mattis Dementia Rating Scale and the Stroop Task were used to assess cognitive decline. MR imaging examinations included T1-MPRAGE, FLAIR, and resting-state fMRI sequences. MPRAGE was segmented to obtain basal ganglia and cingulate cortex volumes. FLAIR was segmented to obtain white matter hyperintensity lesion volume. Resting-state fMRI sequences were used to compare basal ganglia functional connectivity with the cingulate cortex between patients and controls.
RESULTS: Patients with vascular parkinsonism exhibited impaired attention, resistance to interference, and inhibitory control and an increased number of errors on the Stroop Task. They also had higher caudate nucleus and white matter hyperintensity lesion volumes, which were positively correlated (ρ = 0.75, P < .0001). Caudate nucleus functional connectivity with the perigenual anterior cingulate cortex was increased in patients with vascular parkinsonism compared with controls and patients with Parkinson disease, and it was positively correlated with the caudate nucleus volume (ρ = 0.44, P = .016). Caudate nucleus functional connectivity with the posterior cingulate cortex was decreased in patients with vascular parkinsonism compared with controls and negatively correlated with the number of errors on the Stroop test (ρ = −0.51, P = .0003).
CONCLUSIONS: In patients with vascular parkinsonism, cognitive decline could be related to changes of caudate nucleus functional connectivity with the cingulate cortex at resting-state, which may be induced by ischemia-related remodelling.
ABBREVIATIONS:
- BOLD
- blood oxygen level–dependent
- MNI
- Montreal Neurological Institute
- PD
- Parkinson disease
- VP
- vascular parkinsonism
- rsfMRI
- resting-state fMRI
- WMHL
- white matter hyperintensity lesions
Brain lesions related to small-vessel disease may affect the white matter and basal ganglia and can lead to parkinsonism features.1 Vascular parkinsonism (VP) has thus emerged as a secondary parkinsonism subtype that occurs in 3.2/100,000 individuals per year and 2 times more frequently in men than in women.2 Clinically, patients with VP more frequently present with lower body parkinsonism, urinary incontinence, and abnormal pyramidal response.3 Compared with age-matched patients with Parkinson disease (PD) and healthy controls, they also have significantly higher cognitive decline with impaired attention and resistance to interference and reduced inhibitory control.4 Regarding brain morphology, patients with VP are characterized by more frequent basal ganglia lesions5⇓–7 compared with patients with PD and controls. Because attention, resistance to interference, and inhibitory control were related to cingulate cortex function, we hypothesized that basal ganglia functional connectivity with the cingulate cortex could be modified in patients with VP.
Resting-state fMRI (rsfMRI) provides information regarding the functional connectivity of brain structures. This relies on temporal correlation of activity estimated by measuring the blood oxygen level-dependent (BOLD) signal. Seed-driven functional connectivity MR imaging allows determining correlation coefficients between the time course of a seed ROI and the time course of others voxels (seed-to-voxel analysis) or determining correlations between each pair of seed areas (ROI-to-ROI analysis).
We thus aimed to determine whether alteration of basal ganglia functional connectivity with the cingulate cortex could be involved in the cognitive impairment of patients with VP compared with those with PD and healthy controls by using both seed-to-voxel and ROI-to-ROI analyses of rsfMRI sequences.
Materials and Methods
Study Population
Between December 2011 and December 2014, 35 patients (mean age, 79 ± 5 years; male/female ratio, 25:10) with parkinsonism and 27 healthy age-matched controls (mean age, 78 ± 3 years; male/female ratio, 19:8) were prospectively enrolled and underwent brain MR imaging. Inclusion criteria for patients were the following: between 70 and 90 years of age, symptoms of parkinsonism starting after 70 years of age, and a diagnosis of VP or PD. Controls were chosen to match the age distribution of the patients. Exclusion criteria were the same for all subjects (controls and patients) and included the following: a history of head injury, stroke, intracranial bleeding, exposure to neuroleptic drugs, psychiatric comorbidity, and contraindications to MR imaging. A neuroradiologist who was not involved in MR imaging data processing analyzed previous brain MR imaging or CT examinations available on the institutional PACS system before inclusion as well as MR images obtained during the study to rule out stroke and intracranial bleeding sequelae.
Ethics Approval
The institutional review board gave its approval for this study. The experiments were undertaken with the understanding and written informed consent of each subject, and the study complied with the World Medical Association Declaration of Helsinki.
Clinical Assessment
All patients underwent a neurologic evaluation the day of the brain MR imaging examination. Diagnoses were established by an experienced neurologist according to the UK Parkinson's Disease Society Brain Bank criteria8 for idiopathic PD. For VP, the clinical criteria of Zijlmans et al9 (hypokinesia/bradykinesia predominantly affecting the lower body with frontal gait disorder; muscular rigidity; postural instability not caused by visual, vestibular, cerebellar or proprioceptive dysfunction; and resting tremor) were used along with minimal or absent levodopa response and multiple vascular risk factors. Patients were thus classified in 2 groups: 8 patients met the criteria for VP (mean age, 78 ± 2 years; male/female ratio, 7:1) and 27 patients met those of idiopathic PD (mean age, 80 ± 5 years; male/female ratio, 18:9).
The severity of the parkinsonian symptoms was assessed by using the Hoehn and Yahr scale (range, 0–5), the Schwab and England Activities of Daily Living scale (range, 0%–100%), the Movement Disorder Society Unified Parkinson's Disease Rating Scale (range, 0–199),10 and the Short Motor Disability Scale11 (range, 0–17). The educational attainment was also recorded.
Global cognitive efficiency was assessed by the Mini-Mental State Examination score (range, 0–30) and the Mattis Dementia Rating Scale (range, 0–144), involving 5 items (attention, initiation, construction, conceptualization, and memory). To assess executive function, we performed a Victoria Stroop Task.12 It involved 3 conditions: color naming, word reading, and interference, in which a color name is written in a different color. Four colors, red, yellow, blue, and green, were used. Z scores of color naming; word reading; interference; weak interference index (word reading time/color-naming time), which reflects inhibitory abilities when the interference produced by an inappropriate answer is weak; and strong interference index (interference time/color naming time), which reflects inhibitory abilities when the interference produced by an inappropriate answer is strong (ie, classic “Stroop effect”); and the number of errors were recorded.
MR Imaging Protocol
The morning after the clinical assessment, all participants underwent a brain MR imaging on a 3T scanner (Magnetom Skyra; Siemens, Erlangen, Germany). The protocol included a T1-weighted MPRAGE sequence (TR = 1690 ms, TE = 2.54 ms, flip angle = 9°, section thickness = 1 mm, 176 sections, isotropic voxel size = 1 mm3), a FLAIR sequence (TR = 5000 ms, TE = 384 ms, TI = 1800 ms, flip angle = 120°, section thickness = 0.9 mm, 160 sections, voxel size = 0.4 × 0.4 × 0.9 mm3), and a BOLD sensitive functional sequence (TR = 2660 ms, TE = 30 ms, echo-train length = 36, flip angle = 90°, section thickness = 3 mm, in-plane resolution = 2.39 × 2.39 mm, 44 sections, no intersection gap, interleaved acquisition, 200 volumes, acquisition time = 8 minutes). For rsfMRI, acquisitions were started 10 minutes after participants were asked to lie still and awake with their eyes closed.
Structural Data Processing
A neuroradiologist blinded to the patients' clinical diagnoses processed structural T1-MPRAGE data by using SPM8 (http://www.fil.ion.ucl.ac.uk/spm/) running on Matlab 2014a (MathWorks, Natick, Massachusetts). The preprocessing pipeline included the following steps: realignment, segmentation (gray matter, white matter, CSF), normalization in Montreal Neurological Institute (MNI) space, and smoothing with default settings. ROIs around the basal ganglia (caudate nucleus, putamen, and pallidum) were automatically delineated on the average normalized T1-MPRAGE template of all subjects by using the Anatomical Automatic Labeling (SPM8) atlas in MNI space.
At the patient level, we also extracted normalized volume [Normalized Volume in Percentages = 100 × (Brain Structure Absolute Volume in Milliliters/Total Intracranial Volume in Milliliters)] of the caudate nucleus, putamen, pallidum, and cingulate cortex from native T1-MPRAGE by using the algorithm MorphoBox.13 White matter hyperintensity lesions (WMHL) were also semiautomatically delineated on FLAIR images by using MRIcron software (https://www.nitrc.org/projects/mricron) to obtain WMHL volume, which was also normalized according to total intracranial volume.
Resting-State Data Processing
All rsfMRI (n = 53) was preprocessed by a neuroradiologist, blinded to the patients' clinical diagnoses, with SPM8 running on Matlab 2014a. For each participant, the first 5 volumes were discarded to allow equilibration of the magnetic field and the participants' adaptation to the scanning noise.14 The preprocessing pipeline included the following steps: section timing, realignment, coregistration, normalization in MNI-space, and smoothing by using a Gaussian filter with a full width at half maximum of 8 mm. Preprocessed rsfMRI data, structural T1-MPRAGE data, and predefined ROIs were imported in the toolbox Conn (www.nitrc.org/projects/conn). Additional preprocessing included de-noising with voxelwise removal of linear trends over each patient's rsfMRI dataset and temporal low-pass filtering (0.009 Hz < f < 0.08 Hz) to retain low-frequency fluctuations. The BOLD signal of white matter and CSF and movement parameters were used as covariates to remove unwanted physiologic and motion artifact effects.15 First-level analyses (within subjects) then used a weighted General Linear Model for estimation of the Fisher-transformed bivariate correlation coefficients between the seed time-series and each voxel time-series. Functional connectivity measures were tested at the second level (between subjects) by using random effects analyses on a seed-to-voxel basis by using the ROIs defined above. Contrasts among groups (controls, VP, and PD) were generated as 2-tailed t tests with a combination of a voxelwise threshold (uncorrected P < .001) and a cluster-extent threshold (family-wise error rate–corrected P < .05), while controlling for age.16 To explore the potential effect of basal ganglia volumes on functional connectivity, we used normalized basal ganglia volumes secondarily as covariates.
Because Stroop Task performance was related to activity of the anterior cingulate cortex,17 we also performed an ROI-to-ROI analysis between the basal ganglia and the anterior cingulate cortex subregions (subgenual, perigenual, and supragenual). Subregions were segmented on the anterior cingulate cortex AAL atlas region on the basis of the parcellation proposed by Vogt.18 Contrasts between groups were generated as 2-tailed t tests with a combination of a voxelwise threshold (uncorrected P < .001) and a cluster-extent threshold (false-discovery rate–corrected P < .05), while controlling for age.16
Statistical Analysis
Continuous variables are presented as mean ± SD. Three groups were defined according to clinical evaluation: VP, PD, and controls. Patient characteristics were compared by using a nonparametric Kruskal-Wallis test for continuous variables and a Fisher exact test for categoric variables. The Wilcoxon signed rank test was used for post hoc analysis. A P value < .05 was considered statistically significant. The potential relation between clinical scores and MR volumetry or rsfMRI results was assessed by a 2-sided Spearman correlation coefficient (ρ). All statistics were performed with STATA 13.1 software (StataCorp, College Station, Texas).
Results
Study Population Clinical and Volumetric Characteristics
None of the participants were reported to have fallen asleep during the rsfMRI sequence. Of the 62 participants, 4 controls and 5 patients with PD did not remain lying down during the whole rsfMRI sequence due to back pain and/or uncontrolled head motion and were thus excluded from the analysis. In total, we analyzed 53 individual datasets: 23 controls (mean age, 78 ± 2 years; male/female ratio, 17:6), 8 patients with VP (mean age, 78 ± 2 years; male/female ratio, 7:1), and 22 patients with PD (mean age, 80 ± 5 years; male/female ratio, 15:7).
Clinical and MR imaging volumetric characteristics of subjects included in the rsfMRI analysis (n = 53) are reported in the On-line Table. Regarding the Mattis Dementia Rating Scale, patients with VP had significantly lower scores for attention and a lower total score than controls. Regarding the Victoria Stroop Task, patients with VP had significantly lower scores for color naming and word reading and a higher number of errors than controls. Patients with VP also had lower scores for color naming than patients with PD. MR imaging volumetry demonstrated caudate nucleus hypertrophy and higher WMHL volumes in patients with VP, but similar cingulate cortex volumes compared with patients with PD and controls. Caudate nucleus and WMHL volumes were highly correlated (n = 53, ρ = 0.75, P < .0001). There was no significant correlation between the caudate nucleus volume and neuropsychological metrics.
Increased Functional Connectivity in Patients with Vascular Parkinsonism
MNI coordinates of regions with increased functional connectivity from the basal ganglia to the cingulate cortex in patients with VP, on seed-to-voxel analysis, are reported in the Table. Functional connectivity of both the left and right caudate nuclei to the anterior cingulate cortex was increased in patients with VP compared with controls as well as functional connectivity of the right caudate nucleus to the anterior cingulate cortex compared with patients with PD (Fig 1A–C). On ROI-to-ROI analysis, only right caudate nucleus functional connectivity with the bilateral perigenual but not the supragenual anterior cingulate cortex was increased in patients with VP compared with those with PD. Both on seed-to-voxel and ROI-to-ROI analyses, the increased functional connectivity of the right caudate nucleus disappeared when controlling for caudate volume. Indeed, the caudate nucleus volume positively correlated (ρ = 0.44, P = .016) with the functional connectivity between the right caudate nucleus and the perigenual anterior cingulate cortex (Fig 1D).
Basal ganglia functional connectivity with the cingulate cortex in patients with VP
Comparison of the functional connectivity of the right caudate nucleus between patients with vascular parkinsonism and those with Parkinson disease. Contrast is generated as a 2-tailed t test (scale: t = 0.1–5) with a combination of a voxelwise threshold (uncorrected P < .001) and a cluster-extent threshold (family-wise error rate–corrected P < .05), while controlling for age. Increased functional connectivity of the right caudate nucleus to the perigenual anterior cingulate cortex (MNI coordinates: x = 2, y = 44, z = 2) in axial (A), sagittal (B), and coronal (C) planes is observed. Functional connectivity of the right caudate nucleus to the perigenual anterior cingulate cortex positively correlates with normalized caudate nucleus volumes (D).
We did not find any significant correlation between neuropsychological metrics and the right caudate nucleus to perigenual anterior cingulate cortex functional connectivity (all P values > .11).
Decreased Functional Connectivity in Patients with Vascular Parkinsonism
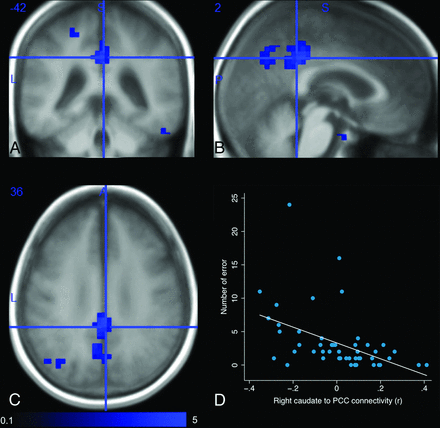
MNI coordinates of regions with decreased functional connectivity from the basal ganglia to the cingulate cortex in patients with VP are reported in the Table. Right and left caudate nuclei functional connectivity with the posterior cingulate cortex and ipsilateral precuneus cortex was decreased in patients with VP compared with controls (Fig 2A–C). The difference did not disappear when controlling for caudate volume. Caudate nucleus functional connectivity with the posterior cingulate cortex was not correlated with the caudate volume (ρ = −0.12, P = .50).
Comparison of the functional connectivity of the right caudate nucleus between patients with vascular parkinsonism and controls. Contrast is generated as a 2-tailed t test (scale: t = 0.1–5) with a combination of a voxelwise threshold (uncorrected P < .001) and a cluster-extent threshold (family-wise error rate–corrected P < .05), while controlling for age. Decreased functional connectivity of the right caudate nucleus to the posterior cingulate cortex (MNI coordinates: x = 2, y = −42, z = 36) in the axial (A), sagittal (B), and coronal (C) planes is observed. Functional connectivity of the right caudate nucleus to the posterior cingulate cortex negatively correlates with the number of errors during the Stroop Task (D).
Right caudate nucleus functional connectivity with the posterior cingulate cortex was negatively correlated with the number of errors on the Victoria Stroop Task (ρ = −0.51, P = .0003) (Fig 2D).
Discussion
The main findings in patients with VP may be summarized as follows: 1) They have impaired attention, resistance to interference, and inhibitory control and an increased number of errors on the Stroop Task; 2) caudate nucleus functional connectivity with the perigenual anterior cingulate cortex was increased, and caudate nucleus functional connectivity with the posterior cingulate cortex was decreased; and 3) caudate nucleus functional connectivity with the perigenual anterior cingulate cortex positively correlated with caudate nucleus volume.
Cognitive Impairment in Vascular Parkinsonism
Vascular parkinsonism is commonly described as a secondary parkinsonism subtype, morphologically characterized by more white matter and basal ganglia lesions than age-matched patients with Parkinson disease.7 However, these unspecific brain lesions may also be encountered in patients with no parkinsonian syndrome, especially in the elderly population. Whether these lesions contribute to parkinsonism or are only concomitant is still unclear; this uncertainty leads to persistent controversy on the reality of this syndrome.19 Clinically, severe cognitive decline has been described as a hallmark in patients with so-called vascular parkinsonism.4,20⇓–22 In agreement, we observed that patients with VP had lower total scores on the Mattis Dementia Rating Scale than healthy controls of similar educational attainment level, reflecting global cognitive impairment. On the Victoria Stroop Task, they specifically had impaired attention and resistance to interference, reduced inhibitory control, and an increased number of errors, findings also concordant with recent studies.3,4 The number of patients receiving levodopa, the dose of levodopa, and the time between last intake and clinical assessment were similar between patients with VP and those with PD. This finding suggests that levodopa medication did not influence cognitive status, as previously reported,23⇓–25 and that it may not account for functional imaging differences in our study.
Caudate Nucleus Functional Connectivity and Relation to Volume
Caudate nucleus functional connectivity with the anterior cingulate cortex has been reported,16,26⇓–28 especially with the perigenual anterior cingulate cortex.26,29 In the present study, we found that caudate nuclei functional connectivity with the perigenual anterior cingulate cortex was increased in patients with VP compared with controls and patients with PD. Caudate nucleus functional connectivity with the posterior cingulate cortex was also decreased in patients with VP compared with controls. While cingulate cortex volumes were similar across study groups, patients with VP exhibited higher caudate volumes. The increased functional connectivity between the caudate nucleus and the perigenual anterior cingulate cortex, moreover, disappeared when controlling for caudate nucleus volume. Rauch et al30 demonstrated that lesions of the anterior cingulate cortex created for treatment of obsessive-compulsive disorder induced caudate nucleus atrophy, which confirmed structural interaction. Inversely, caudate hypertrophy was observed in patients during the subacute phase of ischemic corticostriatal stroke.31 Thus, we observed that the caudate volume was positively correlated with the WMHL volume, a marker of the severity of brain small-vessel disease.1 This correlation seems to indicate that repeat ischemic events observed in patients with VP could induce caudate hypertrophy, which may lead to increased caudate nucleus functional connectivity with the perigenual anterior cingulate cortex at resting-state. The time course of caudate hypertrophy and resting-state functional connectivity increase following ischemic events, however, remains unknown and could be evaluated by longitudinal morphologic and rsfMRI studies.
Relation between Cognitive Impairment and Functional Connectivity Changes
At resting-state, in patients with VP, caudate nucleus functional connectivity with the perigenual anterior cingulate cortex and posterior cingulate cortex was altered, with both the perigenual anterior cingulate cortex and posterior cingulate cortex being part of the default mode network.32 Patients with VP also had concomitant impaired attention on the Mattis Dementia Rating Scale and impaired resistance to interference and inhibitory control as well as an increased number of errors on the Stroop Task.
Concerning attention, increased perigenual anterior cingulate cortex activation has been associated with attentional lapses in healthy subjects.32 Moreover, in normal aging, reduced resting-state connectivity of the posterior cingulate cortex has been correlated with less effective executive function and processing speed.33 Lin et al34 also recently reported that high resting-state posterior cingulate cortex connectivity within the default mode network was associated with low reaction time during attentional tasks. In agreement, we observed that resting-state caudate nucleus functional connectivity with the perigenual anterior cingulate cortex was increased and resting-state caudate nucleus functional connectivity with the posterior cingulate cortex was decreased in patients with VP, both of which could contribute to impaired attention on the Mattis Dementia Rating Scale.
With regard to resistance to interference and inhibitory control, the perigenual anterior cingulate cortex has also been described as a task-negative region in which a deactivation is observed in response to a specific cognitive task. During the Stroop Task,17 a negative BOLD response in the perigenual anterior cingulate region is induced with concomitant increased activity in the supragenual anterior cingulate region.35 Stroop performance was thus negatively correlated with BOLD signal-intensity change in the perigenual anterior cingulate cortex. In patients with VP, increased functional connectivity between the caudate nucleus and the perigenual anterior cingulate cortex at resting-state was observed with concomitant impaired inhibitory control and resistance to interference on the Stroop Task. This finding may suggest incomplete deactivation of the perigenual anterior cingulate cortex during the Stroop Task. Abnormally increased resting-state activity may thus not be sufficiently suppressed during the task, which could result in impaired inhibitory control and resistance to interference.
With regard to response errors, we observed an increased number of errors on the Stroop Task in patients with VP. Increased resting-state default mode network activation was described before response errors.36 Two studies also showed that stop-signal errors are preceded by greater resting-state activity in the perigenual anterior cingulate cortex.37 Although we observed a concomitant increased number of errors on the Stroop Task and increased caudate nucleus functional connectivity to the perigenual anterior cingulate cortex, we did not find any significant correlation between them and could thus not confirm any causal link in patients with VP. However, the number of errors on the Stroop task was negatively correlated with functional connectivity of the caudate nucleus to the posterior cingulate cortex. As discussed above, resting-state connectivity of the posterior cingulate cortex was positively correlated with attentional task performance.34 This correlation suggests that the higher number of errors could be due rather to attention impairment in patients with VP, but it needs confirmatory studies.
Study Limitations
We have to address some limitations in our study. First, we report results in a small population of patients with VP due to the low incidence of the disease, the focus on an elderly population, and the application of restrictive inclusion criteria to limit confounding factors such as exposure to neuroleptic drugs, brain traumatic injury, or stroke. While the observed functional connectivity differences were highly significant and are concordant with brain spatial distribution of cognitive function reported in the literature, correlations with clinical scores were weak. This outcome might be due to underpowered analysis, but cognitive decline could also result from impairment of functional connectivity between structures other than the basal ganglia and cingulate cortex. Second, because we focused on subjects older than 70 years of age, similar study should be performed to confirm these results in younger patients. Third, the correlation between caudate volume and caudate nucleus functional connectivity with the posterior cingulate cortex was insignificant. The posterior cingulate cortex sits at the crossing of multiple connectivity networks,38 with a relatively low contribution from the caudate nucleus.27 Beyond analysis underpowering, low connectivity with the caudate nucleus and interference from other networks could explain why the impact of the caudate nucleus hypertrophy was insignificant or masked. Fourth, we found concomitant increased caudate nucleus functional connectivity with the perigenual anterior cingulate cortex at resting-state and impaired resistance to interference and inhibitory control in patients with VP, which suggest abnormal deactivation during the Stroop Task. This finding should be confirmed by a task fMRI study including pre-, per-, and post-task acquisitions. Finally, we demonstrated that increased caudate nucleus functional connectivity with the perigenual anterior cingulate cortex was related to caudate nucleus hypertrophy in patients with VP. The time course of caudate hypertrophy and increased resting-state connectivity after ischemic events should be evaluated by longitudinal studies.
Conclusions
In patients with VP, cognitive impairment could be related to increased caudate nucleus functional connectivity with the perigenual anterior cingulate cortex and decreased caudate nucleus functional connectivity with the posterior cingulate cortex at resting-state. Increased caudate nucleus–perigenual anterior cingulate cortex functional connectivity positively correlated with caudate nucleus hypertrophy, which was related to WMHL volume, a marker of small-vessel disease. These findings suggest that ischemia-related remodelling may contribute to cognitive decline in patients with VP.
Footnotes
This work was supported by grants from the Centre Hospitalier Regional Universitaire of Montpellier, which was not involved in any step of the study.
References
- Received January 28, 2016.
- Accepted after revision June 5, 2016.
- © 2016 by American Journal of Neuroradiology