Abstract
BACKGROUND AND PURPOSE: Selective ophthalmic artery infusion chemotherapy has improved ocular outcomes in children with retinoblastoma. Our aim was to correlate quantitative tumor reduction and dichotomous therapeutic response with technical and adjunctive factors during selective ophthalmic artery infusion chemotherapy for retinoblastoma. An understanding of such factors may improve therapeutic efficacy.
MATERIALS AND METHODS: All patients with retinoblastoma treated by selective ophthalmic artery infusion chemotherapy at a single center during a 9-year period were reviewed. Only first-cycle treatments for previously untreated eyes were studied. Adjunctive factors (intra-arterial verapamil, intranasal oxymetazoline external carotid balloon occlusion) and technical factors (chemotherapy infusion time, fluoroscopy time) were documented by medical record review. Quantitative tumor reduction was determined by blinded comparison of retinal imaging acquired during examination under anesthesia before and 3–4 weeks after treatment. The dichotomous therapeutic response was classified according to quantitative tumor reduction as satisfactory (≥ 50%) or poor (<50%).
RESULTS: Twenty-one eyes met the inclusion criteria. Patients ranged from 2 to 59 months of age. Adjuncts included intra-arterial verapamil in 15, intranasal oxymetazoline in 14, and external carotid balloon occlusion in 14. Quantitative tumor reduction ranged from 15% to 95%. Six showed poor dichotomous therapeutic response. A satisfactory dichotomous therapeutic response was correlated with intra-arterial verapamil (P = .03) in the aggregate cohort and in a subgroup undergoing treatment with single-agent melphalan at a dose of <5 mg (P = .02). In the latter, higher average quantitative tumor reduction correlated with intra-arterial verapamil (P < .01).
CONCLUSIONS: Intra-arterial verapamil during selective ophthalmic artery infusion chemotherapy is correlated with an improved therapeutic response, particularly when treating with lower doses of single-agent melphalan.
ABBREVIATIONS:
- CIT
- chemotherapeutic infusion time
- DTR
- dichotomous therapeutic response
- ECBO
- external carotid artery balloon occlusion
- FT
- fluoroscopy time
- IAV
- intra-arterial verapamil
- INA
- intra-nasal Afrin
- OA
- ophthalmic artery
- SOAIC
- selective ophthalmic artery infusion chemotherapy
- QTR
- quantitative tumor reduction
Selective ophthalmic artery infusion chemotherapy (SOAIC) has emerged as an important approach to ocular salvage in children with intermediate-to-advanced intraocular retinoblastoma. Numerous variations in neuroendovascular technique have been reported. Temporary or permanent occlusion of the external carotid artery, infusion of verapamil into the ophthalmic artery (OA), and intranasal oxymetazoline or (Afrin) (INA) have all been described as adjunctive methods to optimize ocular hemodynamics.1,2 Known factors associated with failure of intravenous chemotherapy for retinoblastoma include older patient age, greater tumor thickness, vitreal seeds, and subretinal fluid at presentation.3 Tumor response to SOAIC is proportional to the product of tissue chemotherapeutic concentration and the duration of chemotherapeutic exposure.4,5 Each of these factors is influenced by variations in the neuroendovascular technique, particularly adjuncts that modulate ocular blood flow and introduce variations in chemotherapeutic dilution and reflux.
We sought to determine the impact of technical and adjunctive factors on quantitative tumor reduction (QTR) and dichotomous therapeutic response (DTR) as biomarkers of SOAIC therapeutic efficacy. We also evaluated the association of chemotherapeutic infusion time (CIT), a correlate of drug exposure duration, and fluoroscopy time (FT), a surrogate of the difficulty of OA catheterization, with specific therapeutic adjuncts, QTR and DTR.
MATERIALS AND METHODS
The clinical material for this study comprises patients with retinoblastoma who were evaluated and treated at Cincinnati Children’s Hospital Medical Center. This study was approved by the institutional review board at Cincinnati Children’s Hospital Medical Center. Records of all patients with retinoblastoma treated by SOAIC at Cincinnati Children’s Hospital Medical Center during a 9-year period (December 2008 to July 2017) were evaluated. Only the first SOAIC cycles for previously untreated eyes in chemonaive patients were studied to avoid the confounding effects of prior treatment. This approach also optimized our chances of observing measurable changes in QTR because the largest tumor reduction is known to occur with the first SOAIC.6
Baseline tumor features and treatment response were documented by the senior author during ophthalmic examination under anesthesia. During this examination, retinal photographs were obtained with a wide-field retinal imaging system (Retcam; Clarity Medical Systems). All SOAICs were performed by the senior neurointerventionist. SOAIC was conducted by placing the tip of a steam-shaped 1.5F microcatheter (Marathon; Medtronic) directly into the ostium of the OA.1 If a stable ostial microcatheter position could not be established after multiple attempts with alternate microcatheter tip shapes, midsegment OA catheterization was performed with a 1.2F microcatheter (Magic FM; Balt).
Steam-shaping of microcatheter tips was accomplished by inserting a malleable wire template into the microcatheter tip and then shaping the wire template to conform to a curved line drawn through the infraophthalmic internal carotid artery lumen into the OA origin on lateral projections of simultaneously acquired carotid angiograms. The reconfigured microcatheter tip and coaxial wire template were placed into a steam jet for 1–2 minutes. Before removal of the wire template, the microcatheter tip was submerged into and flushed with room temperature saline. Microcatheter tips steam-shaped in this manner generally retained patient-specific curvatures that would support a stable ostial microcatheter position.
In the initial phase of this experience, intra-arterial verapamil (IAV) was selectively administered to patients with a small or constricted OA. In the later phase, IAV was administered to all patients. IAV consisted of 20 µg per kilogram of verapamil (in 2 mL) infused into the internal carotid artery for 2 minutes and 80 µg per kilogram of verapamil (in 8 mL) infused into the OA for 8 minutes. Also, in the initial phase, INA, comprising 2 puffs ipsilateral to the OA, was administered to all patients after establishing general anesthesia. In the latter phase, INA was selectively administered to patients with robust OA perfusion of the nasal mucosa. External carotid artery balloon occlusion (ECBO) was performed as previously described.1 Patients were selected for ECBO if carotid or OA angiography showed continuous or intermittent retrograde OA flow. Patients undergoing SOAIC with a single agent were treated with melphalan or topotecan. SOAIC with single-agent topotecan was performed according to an investigational protocol (NCT01466855). Patients undergoing triple-agent SOAIC were treated with melphalan, topotecan, and carboplatin. Chemotherapeutics and dosing schemes were selected as reported previously.7
Patient age, sex, International Classification of Intraocular Retinoblastoma group, tumor laterality, history of familial retinoblastoma, date of SOAIC, procedure-related adverse events, and chemotherapeutics were documented by electronic medical record review. The date of SOAIC was used to divide cases into early (before May 2015) and late (subsequent to May 2015) epochs. Epochs were defined according to a time point that divided the study period into halves according to the number of the first-cycle SOAICs. Adjunctive (IAV, INA, ECBO) and technical (CIT, FT) factors were documented by electronic medical record review. CIT intervals were designated as brief (<20 minutes), intermediate (20–38 minutes), or prolonged (≥ 39 minutes). FT intervals were designated as brief (<20 minutes), intermediate (20–35 minutes), or prolonged (> 35 minutes).
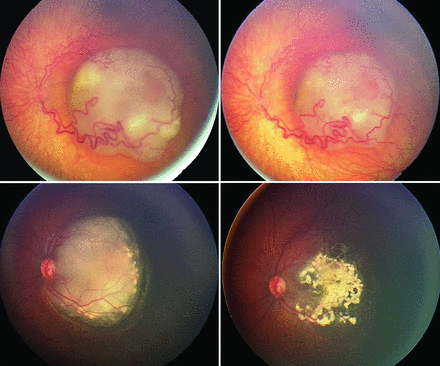
QTR was determined by retrospective estimation of changes in overall tumor size judged by comparison of retinal photographs, fundus maps, and sonographic examinations acquired by ophthalmic examination under anesthesia before and 3–4 weeks after first-cycle SOAIC (Fig 1). All QTR determinations were made by the senior author, an ophthalmologist with fellowship training in vitreoretinal surgery and ocular oncology and >20 years of clinical practice experience. Each QTR determination was made by masking patient identifiers in the presented images. Furthermore, no information about treatment-related technical or adjunctive factors was provided at the time of QTR determination. Consequently, QTR determinations were considered to be blinded with respect to study end points. DTR was categorized as satisfactory (QTR ≥ 50%) or poor (QTR < 50%).
Composite photograph shows 2 eyes of 2 individual patients with macular retinoblastoma displaying substantially different QTRs at 3–4 weeks after the first cycle of selective ophthalmic artery infusion chemotherapy. Analysis of the top pair of photos gives a QTR of 15% and a DTR classified as poor (QTR < 50%). Analysis of the bottom pair of photos gives a QTR of 95% and DTR classified as satisfactory (QTR > 50%).
Preliminary analysis to estimate the potential confounding effect of prior exposure to systemic multiagent chemotherapy was conducted on the entire population of patients undergoing SOAIC treated at the study center during the study period. DTR for first-cycle SOAIC was compared between patients previously exposed to systemic multiagent chemotherapy and patients not previously exposed to systemic multiagent chemotherapy.
For the primary analysis, only first-cycle SOAIC for each enrolled eye was considered. Study inclusion criteria were the following: 1) previously untreated eye, 2) chemonaive patient, and 3) adequate records to retrospectively determine QTR. SOAICs complicated by microcatheter migration during chemoinfusion were excluded. In the primary analysis, primary outcome measures (QTR and DTR) were analyzed in relation to adjunctive factors (IAV, INA, ECBO) and technical factors (CIT, FT) for the aggregate study population and 2 subgroups of first-cycle SOAIC performed with different doses of single-agent melphalan (any dose and <5mg). Secondary analyses included correlation of primary outcome measures (QTR and DTR) with epoch, correlation of technical factors (CIT, FT) with adjunctive factors (IAV, INA, ECBO), and assessment of procedure-related adverse events.
Descriptive analysis was performed to summarize patient demographics, tumor characteristics, and chemotherapeutics. Correlation between categoric variables was evaluated by χ2 or Fisher exact tests. A 2-sample t test or 1-way ANOVA was used to detect differences in average QTR, CIT, and FT between different adjunctive treatment groups and epochs or to assess differences in average QTR between different CIT and FT intervals. Correlation between continuous variables was assessed using the Pearson correlation coefficient. All analyses were performed using SAS software, Version 9.4 (SAS Institute). A P value < .05 was considered statistically significant.
Procedure-related adverse events were assessed for all first-cycle SOAICs performed during the study period.
RESULTS
During December 2008 to July 2017, forty eyes of 35 patients with intraocular retinoblastoma were treated by SOAIC at the study center. Two eyes were excluded because fundus views precluded determination of QTR. Preliminary analysis of the remaining 38 eyes confirmed correlation of prior systemic multiagent chemotherapy with poor DTR (P = .02).
Fifteen eyes previously exposed to systemic multiagent chemotherapy were excluded. One eye was excluded because SOAIC was complicated by microcatheter migration during chemoinfusion. One eye was excluded because of prior local therapy. The remaining 21 eyes of 20 patients composed the aggregate study population. These patients ranged from 2 to 59 months of age (median, 12 months). One of these 20 patients had bilateral retinoblastoma, and both eyes of this patient were enrolled in the study. The single patient with bilateral retinoblastoma was also the only patient in the study with familial retinoblastoma. Laterality was evenly distributed (11 left, 10 right). Eleven eyes were treated in the early epoch, and 10, in the late epoch. The OA was the chemoinfusion artery for all SOAIC treatments. Fourteen first-cycle SOAICs were performed with ECBO, and 7 were performed without ECBO. In 1 case, ECBO was performed because super selective OA angiography demonstrated retrograde OA flow throughout the angiographic cycle. In the remaining 13 cases, ECBO was performed because fluoroscopic monitoring of contrast media injected through the treating microcatheter or superselective OA angiography demonstrated competitive retrograde inflow of unopacified blood into the OA from external carotid artery collaterals as the contrast injection pressure decreased.
The Table and Online Table 1 summarize patient and tumor characteristics. There is a trend toward younger age and male sex in patients achieving satisfactory DTR. Patients treated with ECBO (P < .01) were younger. Low-grade tumors (group B) accounted for a small minority in all comparison groups. Triple-agent chemotherapy had a negative association with INA (P = .03), and single-agent topotecan had a positive association with IAV (P = .04).
Age, sex, history of familial retinoblastoma, and tumor laterality
QTR for the aggregate population ranged from 15% to 95% (average, 59.0%). DTR was poor in 6 and satisfactory in 15 (Online Tables 2–4). DTR and QTR were not significantly associated with sex or age. One-third of patients with poor DTR were cases with single-agent melphalan; one-third, with single-agent topotecan; and one-third, with triple-agent chemotherapy. In the aggregate population, FT ranged from 10 to 76 minutes (average, 29 minutes), and CIT ranged from 12 to 60 minutes (average, 31 minutes).
Online Tables 2 and 3 compare primary outcome measures (QTR and DTR, respectively) between treatment groups differentiated according to adjunctive factors (± IAV, ± INA, ± ECBO). Online Table 4 compares CIT and FT between treatment groups differentiated according to adjunctive factors (± IAV, ± INA, ± ECBO). In the aggregate population, IAV was correlated with satisfactory DTR (P = .03) (Fig 2) but not QTR. Notably, IAV correlated with a late epoch (P = .01), but DTR was not associated with an epoch (Online Table 5). There were 14 first-cycle SOAICs with single-agent melphalan. Five milligrams of melphalan was used in 2 first cycle SOAIC treatments. Analysis of first-cycle SOAIC performed with single-agent melphalan shows correlation of IAV with satisfactory DTR (P = .01) (Online Table 3 and Fig 2) and higher average QTR (P < .01) (Online Table 2) for a melphalan dose of <5 mg. When any melphalan dose is considered, the correlation of IAV with DTR falls just short of statistical significance (P = .06) (Fig 2). In the aggregate population, FT correlated with INA (P < .01) and ECBO (P = .04) (Online Table 4). The difference in average FT between patients with and without ECBO was 11 minutes. The difference in average FT between patients with and without INA was 16 minutes.
The absolute number of satisfactory (black) or poor (white) DTRs for first cycle of SOAIC treatments performed with or without adjunctive IAV is presented for the aggregate study population (A), SOAIC treatments performed with single-agent melphalan at any dose (B), and SOAIC treatments performed with single-agent melphalan at doses of <5 mg (C).
The average CIT was 30.2 and 31.7 minutes in patients with satisfactory and poor DTRs, respectively (Online Table 6). Average FT was 31.9 and 23.3 minutes in patients with satisfactory and poor responses, respectively (Online Table 6). Higher average QTR and satisfactory DTR were associated with decreasing CIT, but differences between CIT intervals were not significant (Fig 3). Similarly, higher average QTR and a satisfactory DTR were associated with increasing FT, but differences between FT intervals were not significant.
The average QTR is presented for the first cycle of selective ophthalmic artery infusion chemotherapy treatment differentiated into brief (<20 minutes), intermediate (20–38 minutes), and prolonged (≥39 minutes) CIT intervals. Error bars indicate ± 1 SD.
Adverse events for 40 first-cycle SOAICs in 35 patients (median age, 16 months) are detailed in Online Table 7. There were no strokes, subclinical cerebral infarctions on brain imaging, or ischemic retinal events. There were 2 transient, subclinical neurovascular events involving reversible vasoconstriction (1 cerebral and 1 ophthalmic) in infants receiving INA.
Transient periorbital erythema secondary to hyperemia was common after IAV and was not counted as an adverse event. Symptomatic periocular edema requiring oral steroids occurred after SOAIC twice, once after IAV. In 2 different cases, ocular hemorrhages were found on ophthalmic examination under anesthesia at 3–4 weeks (1 subretinal and 1 retinal). In both cases, IAV was administered. Neither was associated with prolonged CIT or a wedge microcatheter position, but the retinal hemorrhage was associated with midsegment OA catheterization (Online Table 7).
There were 2 instances of hypotension in infants receiving IAV. Both necessitated resuscitation with blood products. One patient was treated with calcium gluconate to counteract verapamil. There were also 2 instances of bronchospasm, which resolved with microcatheter repositioning and endotracheal albuterol. Postextubation laryngospasm required reintubation in 1 case.
There were 2 access site complications including 1 groin hematoma and 1 transient external iliac artery thrombosis treated by short-term lovenox without sequelae. Both occurred in infants requiring bifemoral arterial access for SOAIC with ECBO.
DISCUSSION
To our knowledge, this is the first study that describes QTR and its derivative DTR, as metrics of SOAIC therapeutic efficacy. QTR uniquely enables one to measure the effect of individual treatments differentiated by technical factors (ie, CIT). In contrast, the ocular salvage rate is determined by the cumulative effect of multiple treatments with variations in technical factors that are difficult or impossible to control. Our focus on first-cycle SOAIC in previously untreated patients allowed us to eliminate confounding effects of prior treatment and isolate the impact of the neuroendovascular technique.
Our study reveals a correlation of IAV with satisfactory DTR. This correlation was significant for the aggregate population and a subgroup treated by SOAIC with <5 mg of single-agent melphalan (Fig 2). Furthermore, the average QTR was higher for first-cycle SOAIC performed with IAV (P < .01) in the latter subgroup. These associations eliminate doubt that the benefit of IAV was due to unequal distribution of differentially effective chemotherapeutics. Although IAV was associated with the late epoch, we found no correlation between DTR and epoch to suggest that the benefit of IAV was attributable to increasing experience of the operating team.
IAV enhancement of therapeutic efficacy could be related to augmentation of ocular perfusion, chemosensitization, or both. Verapamil has potent long-lasting vasodilator actions when administered by the arterial route.8 Because the tumoricidal effect is proportional to tumoral perfusion with chemotherapeutics, vasodilation in target tissue may enhance therapeutic efficacy. Laboratory and clinicopathologic studies have demonstrated that verapamil inhibits membrane-associated glycoproteins, conferring multidrug resistance to retinoblastoma cells and that expression of these glycoproteins by retinoblastoma is associated with treatment failure.9⇓⇓-12 Biochemical studies show that these verapamil-sensitive glycoproteins actively export melphalan, rendering retinoblastoma cells resistant to the chemotherapeutic activity of melphalan.13 Owing to this mechanism, the chemosensitizing potency of verapamil is blunted at higher melphalan doses.14,15 The results of our study are consistent with that paradigm (Fig 2).
The chemosensitizing tissue concentration of verapamil cannot be safely established by oral or intravenous routes, but the pharmacokinetics of superselective intra-arterial administration make it achievable. Others have leveraged intra-arterial administration strategies to commission the chemosensitizing properties of IAV.16,17 Another property of verapamil underlies an anatomically specific advantage unique to the retina. Extensive research has demonstrated that verapamil is actively transported across the blood-retina barrier by retinal pigment epithelium proteins.18,19 Thus, a combination of pharmacokinetic and molecular transport effects may enable chemosensitization with IAV during SOAIC.
In the current series, 2 of 25 children treated with IAV experienced hypotension. Both were infants younger than 12 months of age. Notably, some authors advise against intravenous verapamil administration in infants younger than 12 months of age due to a paucity of safety data.20 Despite such recommendations, intravenous verapamil dosed at 100–200 µg per kilogram for 2 minutes remains the preferred treatment for some types of cardiac dysrhythmia in infants.20 While most agree that the risk of verapamil toxicity is very high during the first 6 weeks of life, there is no evidence that the use of intravenous verapamil in older infants is unsafe.20,21 The incidence of hypotension in this series is substantially lower than that reported in other series of SOAIC.22 In those series, IAV was not given, and procedural hypotension was related to autonomic reflexes associated with OA catheterization.22 It is possible that intraoperative hypotension in the current series was at least partially related to autonomic responses.
INA was not correlated with a therapeutic response in this study. INA has been used to limit perfusion of the sinonasal mucosa with chemotherapeutics during SOAIC. Theoretically, perfusion of the sinonasal mucosa through ethmoidal branches of the OA can cause a steal that reduces delivery of chemotherapeutics to the retina and may cause mucosal injury resulting in epistaxis.23 Two infants receiving INA in the current study experienced procedure-related adverse events related to vasoconstriction (1 cerebral and 1 ophthalmic). Although OA spasm during SOAIC is primarily related to mechanical stimulation, the striking association of INA with increased FT in this study raises the possibility that INA may be a contributory factor and that off-target vasoconstriction caused by INA may interfere with OA catheterization. One patient in the current study demonstrated findings concerning for reversible cerebral vasoconstriction after administration of INA, which cannot be attributed to vessel instrumentation. Reversible cerebral vasoconstriction associated with concomitant administration of oxymetazoline and phenylephrine-containing eye drops during SOAIC has been reported in another patient.24
ECBO is used to optimize ocular hemodynamics during SOAIC because it ensures continuous anterograde OA flow throughout the entire period of chemotherapeutic administration, regardless of microcatheter injection pressure or the patient’s blood pressure.1 The direction of OA flow during SOAIC is assessed by angiography and by subtracted fluoroscopic monitoring of contrast media injected through the treating microcatheter in a manner that simulates chemotherapy administration.1 In children with well-developed external carotid artery-to-OA collaterals, OA flow may be frankly retrograde throughout the entire angiographic cycle. Alternatively, competitive retrograde inflow from external carotid artery collaterals may only be transiently revealed in the delayed phase of angiography as the angiographic injection pressure falls. Under these circumstances, all or some of the administered chemotherapeutic misses the tumor target and refluxes into the cerebral circulation. Another approach intended to overcome competitive flow from external carotid artery collaterals involves administration of chemotherapeutics through the dominant competing external carotid artery collateral, which is frequently the orbital branch of the ipsilateral middle meningeal artery. Advancing a microcatheter deep into such a collateral vessel often produces a pressure gradient that promotes blood flow in the collateral vessel to move toward the external carotid artery, away from the OA. Under these conditions, chemotherapeutic infusion is administered against the direction of arterial flow in the microcatheter-bearing collateral vessel. Moreover, external carotid artery anastomoses with the OA are often distal to the origin of the retinociliary trunk (parent vessel of the central retinal artery and posterior ciliary arteries). This anatomy makes a chemoinfusion administered through the external carotid artery branch countercurrent with blood flow entering the ostium of the OA origin from the internal carotid artery. In order for the chemotherapeutic to reach the retinal circulation under these circumstances, it must overcome a potentially stronger countercurrent flow from the internal carotid artery. As a result, administration of chemotherapy through external carotid artery collaterals trades one source of competitive countercurrent flow with alternative sources of countercurrent flow. We favor ECBO for the management of competitive countercurrent flow encountered during SOAIC because it completely suppresses all sources of countercurrent flow, without introducing new ones. Nonetheless, if the orbital branch of the middle meningeal artery is highly dominant and OA catheterization is challenging, administration of chemotherapy through the orbital branch of the middle meningeal artery may be preferable.
ECBO was not correlated with QTR or DTR in this study. However, because our practice was to perform ECBO in patients with intermittent or continuous OA flow reversal, selection bias likely masked the benefit. ECBO was associated with 11 minutes of additional FT in this study due to the need for fluoroscopy guidance during balloon catheter positioning, inflation, re-adjustment of the balloon during chemoinfusion, and deflation. This amount of added FT may not be significant, depending on the advantage provided by ECBO in any given case. Although there were no adverse events directly attributable to ECBO, our only 2 access site complications occurred in patients undergoing ECBO (Online Table 7). Notably, our method of ECBO relies on bifemoral arterial sheath placement, and patients undergoing ECBO were notably younger. These features raise the possibility that there is a tendency toward retrograde OA flow in younger patients because the ratio of extracranial vascular resistance to cerebrovascular resistance is elevated in early childhood. Indeed, transcranial Doppler studies provide indirect evidence of this.25 It is also possible that age-related changes in OA anatomy may contribute to hemodynamic variations encountered in children. Perhaps expansion of the calvaria and bony orbit in early childhood cause physical displacement of OA anastomoses from corresponding external carotid artery feeders, resulting in developmental involution of anastomoses with maturation of the cranial skeleton. In any case, additional study is warranted to further investigate the safety and potential therapeutic advantage of ECBO.
As reported by others, our approach to SOAIC involves manual infusion of the total chemotherapy dose in a volume of 30 mL.1,23 Although most operators strive to administer the infusion for 30 minutes, adjustment of infusion parameters is often necessary to prevent chemotherapeutic reflux into the internal carotid artery and maximally flood the anterograde target-tissue blood volume. Consequently, CIT varies. Although tumor killing during SOAIC is proportional to the duration of tumor chemotherapeutic exposure, higher average QTR and satisfactory DTR were associated with decreasing CIT in this study, albeit the associations were not statistically significant (Fig 3). It is possible that prolonged CIT with a fixed infusion volume diminishes the therapeutic potency by allowing chemotherapeutics entering the OA to be diluted by a larger volume of inflowing blood.
All except one of the ocular, neurovascular, and cardiovascular adverse events in this series were associated with prolonged FT, and these were predominantly in infants younger than 12 months of age (Online Table 7). The findings emphasize that longer and technically more difficult cases are associated with a generalized increase in procedure-related risks and that infants younger than 12 months of age are particularly at risk. In our study, satisfactory DTR correlated more closely with prolonged FT than brief FT. The results suggest that even children presenting with significant technical challenges realize the full therapeutic benefit of SOAIC. It is notable that the only procedure-related retinal hemorrhage in this series complicated an SOAIC treatment involving midsegment OA catheterization. In our approach to SOAIC, midsegment OA catheterization is generally avoided because we believe that it carries a higher risk of bronchospasm, bradycardia, hypotension, OA spasm, OA dissection, retinal hemorrhage, and retinal ischemia relative to ostial catheterization. Even in the absence of OA spasm, long-segment obturation of the OA by an indwelling microcatheter (as in midsegment catheterization) may create a “wedge dynamic” that pressurizes the retinal circulation during chemoinfusion (leading to retinal hemorrhage) or restricts retinal perfusion (leading to retinal ischemia). Furthermore, procedure-related OA microdissections or intramural hematomas created by excessive OA instrumentation may evolve into a stenosis that thwarts future treatment attempts.
One weakness of this study lies in the retrospective nature of data collection and analysis. Because the details of treatment were obtained by electronic medical record review rather than imaging review, the results are susceptible to documentation inaccuracies and omissions. Another weakness is that most patients received a mixture of different adjunctive therapies, making it difficult to isolate the effect of individual adjuncts. Furthermore, some potentially confounding variations of anatomy and technique (ostial-versus-midsegment OA catheterization) were not considered in our analysis.26 Because midsegment OA catheterization is rarely performed in our practice, we do not think that variations in the OA catheterization technique contributed significantly to our results. Although only 1 case of midsegment catheterization was known to occur, our study methodology did not allow us to systematically classify cases according to catheterization technique. Investigation of the catheterization technique would have required analysis of OA angiograms rather than review of reports contained in the electronic medical record. Future studies should address the potential impact of the OA catherization technique. Although the current study suggests IAV enhancement of therapeutic efficacy, the study cohort is small and further study is necessary to determine whether the added therapeutic benefit outweighs the increased risks of drug-induced hypotension.
CONCLUSIONS
In children undergoing SOAIC for intraocular retinoblastoma, IAV is safe and may increase the probability of a satisfactory therapeutic response, particularly in those treated with <5 mg single-agent melphalan. Procedural hypotension occurs in a minority of infants and is correctable with calcium gluconate and/or volume resuscitation. INA is not associated with measurable benefit and may produce off-target vasoconstrictive effects that lead to adverse events, particularly in infants. Although ECBO did not demonstrate a therapeutic advantage in this study, selection bias may have confounded our assessment.
Footnotes
Disclosures: Sudhakar Vadivelu—UNRELATED: Consultancy: Alcyone Lifesciences. Zelia M. Correa—UNRELATED: Consultancy: Castle Biosciences.
Paper previously presented, in part, at: Annual Meeting of the Society of Interventional Radiology, March 17–22, 2018; Los Angeles, California, abstract No. 527.
References
- Received June 25, 2020.
- Accepted after revision September 4, 2020.
- © 2021 by American Journal of Neuroradiology