Abstract
BACKGROUND AND PURPOSE: Distribution of intracranial hemorrhage in term and late-preterm neonates is relatively unexplored. This descriptive study examines the MR imaging–detectable spectrum of intracranial hemorrhage in this population and potential risk factors.
MATERIALS AND METHODS: Prevalence and distribution of intracranial hemorrhage in consecutive term/late-preterm neonates who underwent brain MR imaging between January 2011 to August 2018 were assessed. MRIs were analyzed to determine intracranial hemorrhage distribution (intraventricular, subarachnoid, subdural, intraparenchymal, and subpial/leptomeningeal), and chart review was performed for potential clinical risk factors.
RESULTS: Of 725 term/late-preterm neonates who underwent brain MR imaging, intracranial hemorrhage occurred in 63 (9%). Fifty-two (83%) had multicompartment intracranial hemorrhage. Intraventricular and subdural were the most common hemorrhage locations, found in 41 (65%) and 39 (62%) neonates, respectively. Intraparenchymal hemorrhage occurred in 33 (52%); subpial, in 19 (30%); subarachnoid, in 12 (19%); and epidural, in 2 (3%) neonates. Twenty infants (32%) were delivered via cesarean delivery, and 5 (8%), via instrumented delivery. Cortical vein thromboses were present in 34 (54%); periventricular or medullary vein thromboses, in 37 (59%); and cerebral venous sinus thrombosis, in 5 (8%). Thirty-seven (59%) had elevated markers of coagulopathy (international normalized ratio > 1.2, fibrinogen level < 234), 9 (14%) had a clinically meaningful elevation in the international normalized ratio (>1.4), and 3 (5%) had a clinically meaningful decrease in the fibrinogen level (<150). Three (5%) neonates had thrombocytopenia (platelet count < 100 × 103/μL).
CONCLUSIONS: While relatively infrequent, there was a wide distribution of intracranial hemorrhage in term and late-preterm infants; intraventricular and subdural hemorrhages were the most common types. We report a high prevalence of venous congestion or thromboses accompanying neonatal intracranial hemorrhage.
ABBREVIATIONS:
- GA
- gestational age
- ICH
- intracranial hemorrhage
- INR
- international normalized ratio
- IPH
- intraparenchymal hemorrhage
- IVH
- intraventricular hemorrhage
- SWAN
- susceptibility-weighted angiography
Intracranial hemorrhages (ICHs) in term (gestational age [GA] ≥ 37 weeks) and late-preterm (GA = 34–36 weeks) neonates can range in clinical manifestations from asymptomatic to potentially devastating.
In preterm neonates, intraventricular hemorrhage (IVH) is the most common ICH type due to immature vessels of the germinal matrix.1-3 In older neonates, however, ICH is less common and differs in location, etiology, and outcome.4 A case-control study of infants born in the Northern California Kaiser Permanente Medical Group identified 20 cases of neonatal ICH with a population prevalence of 6.2 in 100,000 live births, but without investigations into a specific intracranial compartment.5 The clinical presentation of the term neonate with ICH might include encephalopathy, strokes, seizures, apnea, and respiratory distress.5-7
There are conflicting data regarding the association of ICH with traumatic or instrumented delivery via assistance with vacuum or forceps.5,8-11 One Swedish study of vacuum-assisted vaginal delivery of term neonates described 9-fold increased odds of ICH, with protracted extractions compared with extractions adhering to safety recommendations.12 In another study, instrument-assisted birth was not associated with higher odds of intracranial hemorrhage or hemorrhagic stroke.5
There are also conflicting data on the association of coagulopathy with neonatal ICH.6,7 For example, 1 small case series of 17 neonates with subpial hemorrhage, a rare neonatal ICH pattern with hemorrhage interposed between the brain surface and pia mater, observed a relatively high incidence of acute thrombocytopenia and coagulation abnormalities.6 However, in another small series of 7 neonates with subpial or subarachnoid hemorrhage, none had coagulopathy.7
Limited data exist on the prevalence and location of intracranial hemorrhage in term and late-preterm neonates, and less is known about potential risk factors for these hemorrhages. This study describes the spectrum of ICH in late-preterm and term neonates from a large cohort at a single institution.
MATERIALS AND METHODS
Study Design
This was a retrospective descriptive study of neonates who underwent brain MR imaging at Lucile Packard Children’s Hospital at Stanford from January 2011 to August 2018. The study and waiver of consent were approved by the Stanford University Institutional Review Board. Clinical and radiographic data were de-identified.
Participants
The following served as the inclusion criteria: term (GA ≥ 37 weeks) or late-preterm (GA ≥ 34 weeks) neonates with brain MR imaging that included both anatomic scans and iron-sensitive T2* imaging (gradient-echo or susceptibility-weighted angiography [SWAN]) obtained before 45 weeks postmenstrual age. Patients with nondiagnostic brain MR imaging (eg, motion degradation or other artifacts) and lack of T2* MR imaging were excluded. Subjects were identified from a database of patients in the Lucile Packard Children’s Hospital at Stanford Neonatal Intensive Care Unit who underwent brain MR imaging.
Hemorrhage Locations
The ICH location was based on the following criteria: 1) intraventricular hemorrhage, hemorrhage in the cerebral ventricles; 2) subdural hemorrhage, hemorrhage in the subdural space between the dura mater and arachnoid mater; 3) subarachnoid hemorrhage, hemorrhage in the subarachnoid space between the pia mater and arachnoid mater; 4) subpial hemorrhage, hemorrhage overlying the cortex or between the cortical surface and the pia mater; and 5) intraparenchymal hemorrhage (IPH), hemorrhage in the brain parenchyma. Small blood products in the posterior fossa and tentorium typically present after birth were not considered pathologic ICH.
Clinical Information
Chart review was conducted for the following: birth weight, GA, maternal age, method of delivery, instrumented delivery, Apgar scores, indication for imaging, underlying congenital heart disease, history of extracorporeal membrane oxygenation, history of seizures, and coagulation laboratory values. Normal value ranges for the laboratory where the coagulation studies were performed were 234–395 mg/dL for fibrinogen levels and 0.9–1.2 for the international normalized ratio (INR).
Imaging Technique and Analysis
All patients underwent brain MR imaging at either 1.5T (Signa; GE Healthcare) or 3T (Discovery MR750; GE Healthcare). The routine infant brain MR imaging protocol included either a T2* axial gradient-echo (TR = 500–600 ms, TE = 20–30 ms, section thickness = 5 mm, and skip = 1.5 mm) or SWAN (TR = 50–70 ms, TE = 45–25 ms, section thickness = 4 mm, 0 skip). Other imaging sequences included T1 FLAIR, T1 spoiled gradient-recalled, T2 FSE, DWI, and arterial spin-labeling.
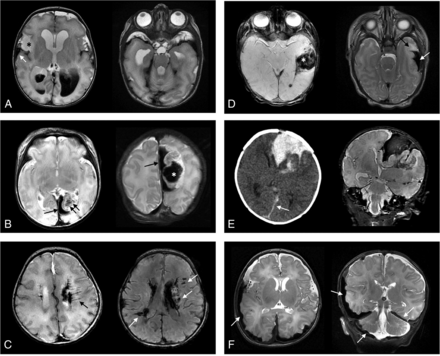
Two board-certified neuroradiologists (E.T. and K.W.Y.), blinded to clinical information, independently reviewed the brain MRIs and cataloged hemorrhage distribution. Additionally, the reviewers recorded the presence of lesions suspicious for venous thromboses, eg, thick, linear, and expansile T2* lesions that followed the anatomic distribution of medullary/periventricular or cortical veins or filling defects/flow gaps associated with dural venous sinus thromboses. Any disagreement between the reviewers was resolved by a consensus review among the 2 neuroradiologists and 2 child neurologists (A.G.S.K., Q.-Z.Y.). Examples of ICH categorization are shown in Fig 1.
Examples of various anatomic distributions of intracranial hemorrhages in term or late-preterm neonates. A, IVH and associated ventriculomegaly are present. Tentorial subdural hemorrhage is also present, as well as hemorrhage overlying the bilateral temporal poles. Right temporal lobe swelling (asterisk) is present and associated with subpial hemorrhage (arrow). B, Left occipital subpial hemorrhage (single arrow) is present, as well as left parieto-occipital lobe parenchymal hemorrhage (asterisk). Linear low signal intensities (double arrows) suggest either venous congestion or thromboses. C, Intra- and periventricular hemorrhages are present. Linear intensities (black arrow) and nodular intensities (white arrows on T2* imaging) suggest a perimedullary vein congestion and/or thromboses. D, Subpial hemorrhage is seen (white arrow) with subjacent temporal lobe deformity and edema (black arrow). On T2* imaging, parenchymal hemorrhage is also seen (asterisk), as well as punctate intraventricular blood products. E, Combined left frontal subpial and parenchymal hemorrhages are shown on CT and MR imaging. Small falcine subdural hemorrhage is also shown (arrow). F, Bilateral cerebral convexity, posterior fossa, and tentorial subdural hemorrhages (arrows) are present in a neonate with hemophilia.
Statistical Analysis
Descriptive statistics included mean (SD) for normally distributed variables and median and interquartile ranges (IQRs) for non-normally distributed variables.
RESULTS
Hemorrhage
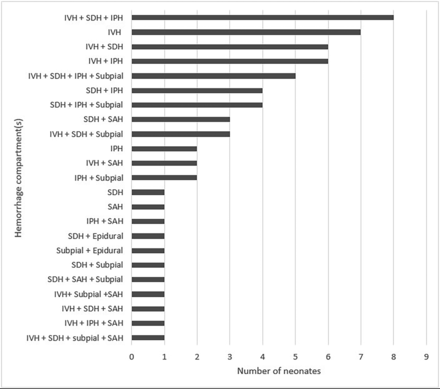
Of 1542 neonates who underwent brain MR imaging between January 2011 and August 2018, seven hundred twenty-five neonates (median day of life = 5.0 days, IQR = 3.0–8.5) met the inclusion criteria: 576 term (GA ≥ 37 weeks) and 149 late-preterm neonates (GA = 34–36.9 weeks). Of 725 neonates, 63 (9%) were found to have ICH: 47 of the 576 (8.2%) term neonates and 16 of the 149 (10.7%) late-preterm neonates. Table 1 summarizes demographics and clinical features. Fifty-two neonates (83%) had >1 type of hemorrhage. IVH and subdural hemorrhage were the most common hemorrhage locations, occurring in 41 (65%) and 39 (62%), respectively. IPH occurred in 33 (52%); subpial hemorrhage, in 19 (30%); SAH, in 12 (19%); and epidural hemorrhage, in 2 (3%) (Figs 2 and 3). T2* lesions suspicious for venous thromboses were identified in more than half of the subjects (Table 2).
Sample characteristics
Distribution of hemorrhages by CNS location. SDH indicates subdural hemorrhage.
Distribution of hemorrhages by CNS location, subdivided by a combination of compartments when multiple compartments were involved. SDH indicates subdural hemorrhage.
Venous thromboses and thrombocytopenia/coagulopathy in neonates with ICH
Clinical Features
Thirty-seven (59%) subjects had elevated markers of coagulopathy (INR > 1.2, fibrinogen level <234 mg/dL). Nine (14%) had a clinically meaningful elevation in the INR of >1.4, and 3 (5%) had a clinically meaningful decrease in fibrinogen levels of <150 mg/dL (Table 2). Three (5%) neonates had platelet counts of <100 × 103/μL. Four neonates were found to have possible genetic etiologies of coagulopathy. One was heterozygous for a factor V Leiden mutation, 1 was heterozygous for prothrombin 20210A mutation, 1 was homozygous for the MTHFR mutation, and 1 was found to have factor VIII hemophilia.
Delivery was instrumented in 5 subjects: 4 with vacuum-assisted delivery and 1 with forceps-assisted delivery. Eight (13%) had congenital heart disease, and 2 (3%) had undergone extracorporeal membrane oxygenation before MR imaging.
The indications for MR imaging were clinical suspicion of hypoxic-ischemic encephalopathy in 11 (17%), seizures in 15 (24%), and abnormal findings on head sonography in 20 (32%). Five subjects (8%) had sepsis or meningitis. Most patients (n = 56, 89%) were discharged home, 5 (8%) were transferred to an outside facility, and 2 (3%) died. Both of the 2 patients who died had chromosomal abnormalities and congenital heart disease.
DISCUSSION
We observed a wide range in ICH distribution in term and late-preterm neonates, with most presenting with multicompartment hemorrhages and intraventricular hemorrhage and subdural hemorrhage representing the most common hemorrhage locations. To date, this is the first study to systematically categorize ICH in term and late-preterm neonates at a large quaternary care pediatric center.
Clinical presentation and indications for MR imaging in our cohort are concordant with prior neonatal studies that examined ICH,5,6 including clinical suspicion of hypoxic-ischemic encephalopathy, seizures, and abnormal findings on head sonography. Intraventricular hemorrhage has been widely studied in premature neonates and remains the most common ICH type affecting preterm infants.1-3 Our sample, although a single-center experience, suggests that IVH is also a common ICH presentation in term and late-preterm neonates, occurring in 65% (n = 41) of those with ICH. However, given the multicompartment ICH distribution in most of our neonates with ICH (83%), we postulate pathophysiology or hemodynamic dysregulation that is distinct from the germinal matrix fragility typically implicated in preterm neonates (Figs 2 and 3). Similarly, a recent small cohort study of 17 neonates with subpial hemorrhages also found concomitant hemorrhages in other brain locations, eg, IPH or IVH.6
Although the relationship between ICH and traumatic or instrumented delivery via vacuum assistance or forceps remains debated, most studies report low rates of ICH with instrumented delivery.5,8-11 Similarly, we report a low rate of instrumented delivery, occurring in only 5 of the 63 subjects (8%). Prior studies have identified an association between fetal distress via emergent cesarean delivery: bradycardia, decelerations, and low Apgar scores with neonatal ICH. However, the rates of delivery by cesarean birth in our sample were similar to those in the general population at 32%, with 9 (45%) scheduled and 11 (55%) for urgent indications; and the median Apgar score at 5 minutes was 9 (IQR = 7–9).13
One potential contributor to neonatal ICH is coagulopathy, despite conflicting data in the literature.6,7 While most (59%) of our neonates with ICH had elevated coagulopathy markers based on the laboratory cutoff values, only 14% had a clinically relevant elevation in the INR of >1.4, and only 5% had either a clinically relevant decrease in platelet count of <100 × 103/μL or in fibrinogen levels of <150 mg/dL. A small minority had genetic etiologies of coagulopathy, including 1 patient with hemophilia. Nevertheless, coagulopathy and thrombocytopenia represent important modifiable potential contributors, whereby early identification and correction of coagulopathy, along with maintenance of homeostasis and hemodynamic stability, are key in the management of critically ill neonates.
Most interestingly, T2* lesions suspicious for periventricular/medullary vein and cortical vein thromboses were common in our sample, occurring in 59% and 54%, respectively, with 37% having both (Table 3). Despite some reports of neonatal IPH associated with cortical vein thrombosis,14 studies on neonatal venous thromboses are sparse. A study of subpial hemorrhages in 17 neonates also found a high incidence of enlarged perimedullary veins (65%–76%) and suggested that medullary venous congestion or thromboses may account for these hemorrhages.6 While the mechanisms for periventricular/medullary or cortical vein thromboses remain unknown, it is possible that they represent sequelae of ICH and associated venous hypertension and congestion or primary/exacerbating contributors to hemorrhage and hemodynamic dysregulation in inflammatory or procoagulant states.
Thromboses and coagulopathy by bleed location
The 2 patients who died in this study each had chromosomal abnormalities and congenital heart disease; thus, ICH was not considered a major or primary contributor to mortality. Long-term neurodevelopmental outcomes are beyond the scope of this study but remain important points of future investigation.
This study has several limitations, primarily the sample size and selection bias inherent in a single quaternary pediatric center. Although this study represents the largest cohort of term or late-preterm neonatal ICHs to date, the sample in each ICH category remains small, which limits group comparisons for potential contributing risk factors or for outcome analysis. The relatively higher prevalence of ICH in our sample compared with prior studies5 may relate to selection bias in our cohort at a quaternary, referral center or a high rate of brain MR imaging use that facilitated ICH detection unique to our academic practice.
CONCLUSIONS
While ICH in preterm infants is well-studied, it is less explored in term or late-preterm neonates. To date, this is the first and the largest study to systematically categorize ICH in this population. We observed a wide range in ICH distribution in term and late-preterm neonates, with most neonates presenting with multicompartment hemorrhages, which might reflect global hemodynamic dysregulation. Periventricular and cortical vein thromboses were common; instrumented delivery was less frequent; and despite abnormal coagulopathy laboratory markers in >50% of the patients with ICH, clinically meaningful coagulopathy was less frequent. Further investigation is needed to determine the association of ICH subtypes in term and late-preterm neonates with later neurodevelopmental outcomes.
Footnotes
A. G. Sandoval Karamian is supported by a grant from the American Epilepsy Society; V.L. Rao is supported by the Stanford MedScholars Fellowship.
Disclosure forms provided by the authors are available with the full text and PDF of this article at www.ajnr.org.
References
- Received February 20, 2022.
- Accepted after revision July 27, 2022.
- © 2022 by American Journal of Neuroradiology