Abstract
BACKGROUND AND PURPOSE: CT is considered the standard reference both for quantification and characterization of carotid artery calcifications. Our aim was to investigate the relationship among different types of calcium configurations detected with CT within the plaque with a novel classification and to investigate the prevalence of cerebrovascular events.
MATERIALS AND METHODS: Seven hundred ninety patients (men = 332; mean age, 69.7 [SD, 13] years; 508 symptomatic for cerebrovascular symptoms and 282 asymptomatic) who underwent computed tomography of the carotid arteries were retrospectively included in this institutional review board–approved study. The plaque was classified into 6 types according to the different types of calcium configurations as the following: type 1, complete absence of calcification within the plaque; type 2, intimal or superficial calcifications; type 3, deep or bulky calcifications; type 4, adventitial calcifications with internal soft plaque of <2 mm thickness; type 5, mixed patterns with intimal and bulky calcifications; and type 6, positive rim sign.
RESULTS: The highest prevalence of cerebrovascular events was observed for type 6, for which 89 of the 99 cases were symptomatic. Type 6 plaque had the highest degree of correlation with TIA, stroke, symptoms, and ipsilateral infarct for both sides with a higher prevalence in younger patients. The frequency of symptoms observed by configuration type significantly differed between right and left plaques, with symptoms observed more frequently in type 6 calcification on the right side (50/53; 94%) than on the left side (39/46; 85%, P < .001).
CONCLUSIONS: We propose a novel carotid artery plaque configuration classification that is associated with the prevalence of cerebrovascular events. If confirmed in longitudinal analysis, this classification could be used to stratify the risk of occurrence of ischemic events.
ABBREVIATION:
- IPH
- intraplaque hemorrhage
Ischemic stroke is one of the leading causes of mortality and disability worldwide.1 Several studies have demonstrated that atherosclerosis involving the carotid arteries is responsible for a significant portion of acute ischemic strokes (up to 25%).2,3 In the past 20 years, the role of carotid plaque imaging in risk stratification of ischemic stroke has evolved considerably. Several recently published articles suggest that the mere degree of stenosis, considered the only criterion for risk stratification at a time when advanced imaging was not available, is not the best parameter to predict the stability and vulnerability of the plaque and its potential for causing cerebrovascular events.2,4,5 Therefore, there has been great emphasis on identifying additional imaging features that can be used to select the best therapeutic approach.
A number of parameters have been identified that are associated with an increased risk of plaque rupture, including intraplaque haemorrhage,6,7 lipid-rich necrotic core,8 rupture of the fibrous cap, and contrast plaque enhancement.9 Other features such as a thick fibrous cap have been linked to a reduced risk of stroke. The role of calcium, present in 50%–60% of carotid plaques, is controversial,10 and the relationship between the amount and topographic distribution of vascular wall calcifications and plaque vulnerability have not been evaluated. Some studies have found that carotid plaque calcification is associated with plaque stability and may be considered a protective plaque feature,11⇓⇓-14 while others have found that it could represent an independent marker of ischemic symptoms.15 The significance of calcifications and their distribution in carotid artery plaques are controversial,15⇓-17 and it is unclear whether the level of carotid calcification should be considered an element of plaque stability or instability. Eisenmenger et al18 proposed a model that includes the positive rim sign, maximum soft-plaque thickness, the NASCET stenosis, and ulceration to predict intraplaque hemorrhages (IPHs) on computed tomography (CT). A positive rim sign was defined as adventitial calcification (<2 mm thick) with internal soft plaque (>2 mm thickness). This model had excellent IPH prediction with an area under the curve of 0.94.18
Calcium in a carotid artery plaque can be identified with different imaging techniques. Among these, CT is considered the standard reference both for quantification and characterization of carotid artery calcifications.19⇓-21 Our hypothesis is that it is possible to classify the CTA patterns of calcifications in the carotid plaque by identifying subgroups that can be more frequently associated with the presence of symptomatic cerebrovascular events. We have, therefore, developed a novel classification based on the different calcium configurations.
In this study, we identified various carotid artery plaque subgroups on the basis of their morphology as visualized on CTA and assessed their clinical significance in a large cohort of symptomatic and asymptomatic patients.
MATERIALS AND METHODS
Study Design and Patient Population
Institutional review board approval was obtained, and informed consent was waived because of the retrospective nature of the study. This 2-center retrospective study was performed in 2 tertiary centers (Azienda Ospedaliera Universitaria di Cagliari [site A] and Stanford University Hospital [site B] between March 2013 and May 2020. All examinations were performed according to established standard-of-care CTA protocols. Patient characteristics including age, sex, clinical symptoms, medical history, and clinical outcomes were recorded.
Patients 18 years of age or older with bilateral carotid artery plaques (thickness of ≥1.5 mm, according to the Mannheim intima-media thickness consensus) were included.22 Excluded were subjects younger than 18 years of age and those in whom the CTA was performed for reasons other than suspected atherosclerotic disease (ie, dissection) or other etiologies for ischemic stroke such as a cardiac embolic source, embolism from the thoracic aorta, and vertebrobasilar artery disease. The degree of stenosis was measured according to the NASCET criteria.23 We also excluded patients with other neurologic conditions such as brain neoplasms, demyelinating disease, and so forth.
According to a standardized protocol, CTA of the carotid artery was obtained in asymptomatic patients when carotid sonography showed significant stenosis (>50% measured according to the NASCET criteria) or features of plaque vulnerability (ulcerations, irregular surface) and when carotid sonography could not adequately assess the degree of stenosis and plaque characteristics because of anatomic conditions. Moreover, all subjects presenting with acute cerebrovascular events underwent CTA of the carotid arteries at the time of their acute CT of the head.
Definition of Symptomatic
Patients were classified by the treating neurologist using the Trial of Org 10172 in Acute Stroke Treatment (TOAST) criteria.24 Consequently, a patient was considered symptomatic after experiencing a TIA or ischemic stroke in either cerebral hemisphere. TIA was considered a brief (<24 hours) episode of neurologic dysfunction such as hemiparesis, hemisensory loss, dysarthria, dysphasia, or monocular blindness. If the episode of neurologic dysfunction exceeded 24 hours, it was classified as a stroke. A lacunar stroke was defined as a stroke causing one of the traditional clinical lacunar syndromes without evidence of cerebral cortical dysfunction. Such patients also had normal CT/MR imaging findings or a relevant brain stem or subcortical hemispheric lesion with a diameter of <0.5 cm.25 A patient was considered symptomatic within a time window of up to 3 months after symptom onset. For our model of analysis, we considered both carotid arteries in each patient: The symptomatic carotid was the one ipsilateral to the symptoms and the asymptomatic carotid was the contralateral one.
CT Technique
Due to the range of time for data collection and the bicenter approach, CTA of the carotid arteries was performed with multiple scanner technologies according to a standardized protocol. As a general approach, patients were placed in the supine position with the head tilted back to prevent dental artifacts. The coverage was from the aortic arch to the carotid siphon in a caudocranial direction.
Plaque Calcification Configuration
Carotid plaques were classified into 6 subgroups based on calcium configurations (Fig 1):
Type 1: complete absence of calcification within the plaque
Type 2: intimal or superficial calcifications
Type 3: deep or bulky calcifications
Type 4: adventitial calcifications with internal soft plaque of <2-mm-thickness; negative rim sign
Type 5: mixed patterns with intimal and bulky calcifications
Type 6: positive rim sign.
Scheme showing the different types of configurations with the corresponding CTA findings.
A superficial calcification was defined as a calcified nodule located at the intimal-lumen interface or close to the lumen, whereas a deep calcification was defined as a calcified nodule located at the media/adventitia border or close to the adventitia. The presence of both deep and superficial calcifications was regarded as a mixed category.26 Bulky calcification was defined as calcification measuring >2 mm thick without associated thin adventitial calcification.18
The classification was performed by 2 radiologists; in the case of conflicts between the readers, the decision was made by a third senior reader expert in vascular imaging. Interobserver and intraobserver (within a 2-month timeframe) agreement was recorded. Interactive window/level settings were usually set at W850:L300.27 This approach was adopted because calcifications may show variable edge and halo blurring depending on the window level, which could change the size (expressed in millimeters) of the calcification; therefore, we opted for this static approach.
Statistical Analysis
Continuous variables were presented as mean (SD) or median (with interquartile range) values. Categoric variables were described as frequency rates and percentages. Comparisons of continuous data were performed using the independent samples t test or Mann-Whitney U test. Shapiro-Wilk tests were used to check continuous variables for normal distribution. Categoric variables were compared using a χ2 test or Fisher exact test as appropriate. Comparisons between groups were performed using a 1-way ANOVA for continuous variables with normal distributions; the Kruskal-Wallis test was used for continuous variables with non-normal distributions. A Tukey multiple comparison test was performed to determine statistically significant changes between each group. Inter-/intraobserver agreement was measured using a weighted Cohen κ analysis. A P value < .05 was considered statistically significant. The Pearson ρ was used to correlate types of plaque with traditional risk factors, infarct, stroke, symptoms, and degree of stenosis. All statistical analyses were performed using SPSS Statistics, Version 25 (IBM).
RESULTS
Baseline Characteristics
The study included 790 patients (men = 42%, women = 58%; mean age, 69.7 [SD, 13] years; age range, 23–102 years) with bilateral plaques, 508 symptomatic and 282 asymptomatic. Clinical and demographic characteristics are summarized in Table 1. The mean degree of stenosis was 61% in the symptomatic patients and 30% in the asymptomatic ones. Plaque on the right side was 39%; and on the left side, 41%.
Demographic and risk factors
Plaque Calcification Type and Symptoms
The crosstabulation among the types of calcifications is given in Table 2. The frequency of symptoms observed by configuration type significantly differed between right and left plaques, with symptoms observed more frequently in type 6 calcification on the right side (50/53; 94%) than on the left side (39/46, 85%; P < .001).
Crosstabulation between calcified plaque on the right and left sides
We found that the absence of calcification on one side is frequently associated with absence of calcification on the contralateral side. However, the weighted overall Cohen κ value was poor, with a value of 0.297. We also tested the inter- and intraobserver concordance; the values are summarized in the Online Supplemental Data.
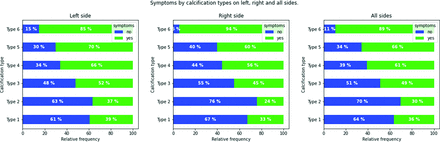
In the analysis of the correlations among the types of calcifications and symptomatic status (by considering 1580 sides), the highest prevalence of symptomatic plaques was observed for type 6, in which 89 of the 99 plaques were symptomatic. A complete summary of the findings is shown in Fig 2.
Bar plots in symptomatic and asymptomatic subjects according to the plaque configuration for the total plaque count (A), the right side (B), and left side (C).
We also checked the impact of side by analyzing the association between the right/left carotid artery plaque configurations and the presence of symptoms (right stroke, left stroke/absence of stroke for asymptomatic patients); the results are summarized in Fig 2. The Pearson χ2 test showed a statistically significant difference for different types of configurations for both right and left plaques (P < .001). Figure 3 shows the relationship among different plaque configurations on the right/left side versus the measured risk factors.
Bar plots in symptomatic and asymptomatic subjects according to the plaque configuration for the total on the right (upper row) and left (lower row) sides for cardiovascular risk factors. CAD indicates coronary artery disease; Y, yes; N, no.
There was an increasing correlation from type 1 to type 6 plaque configurations with symptoms, stroke, ipsilateral infarct, TIA, and NASCET degree of stenosis for both sides. In particular, the highest degree of correlation was found for ipsilateral infarct, ranging from −0.067 for type 1 to 0.21 for type 6 on right side and from −0.11 for type 1 to 0.21 for type 6 on left side (see Fig 4 for further details). No other traditional risk factors for cerebrovascular diseases reached the same degree of correlation (Fig 4). Moreover, as observed in the Online Supplemental Data, by plotting the types of calcifications with age and degree of stenosis, type 6 plaque configuration was more common in younger patients, with a bimodal distribution for stenosis degree (mild and severe) on both sides.
The Pearson ρ correlogram shows the correlation test among the different demographic variables and the calcification types. Each box represents a correlation test, the blue values represent negative correlations, and the red values represent positive associations. CAD indicates coronary artery disease; L, left; R, right.
DISCUSSION
In the present study, we grouped the different types of calcium configurations we encountered in our daily clinical practice by proposing a novel classification system to verify whether some configurations are more likely to be associated with cerebrovascular events.
The analysis of the association between the types of calcifications and the presence of symptoms (by considering 1580 sides) showed that the highest risk of cerebrovascular events is seen for type 6, in which 89 of the 99 patients were symptomatic. These results were also confirmed by the correlation analysis, in which type 6 had the highest degree of correlation with symptoms, stroke, TIA, and ipsilateral infarct. Our results also confirm that the presence of a positive rim sign is significantly associated with cerebrovascular events, but from the analysis of the other ORs, it is quite obvious that the different types of calcium configurations play a different role, with an increasing OR according to the analyzed class. Moreover, after analyzing the relative frequency of plaque configurations among different ages and degrees of stenosis,28,29 type 6 was more frequent in younger patients, with a bimodal distribution with regard to the NASCET degree of stenosis (mild and severe).
The type 1 class identifies those carotid artery plaques without calcification within the plaque, and somewhat surprisingly, we found that plaques without any calcium have an OR of 0.5, which suggests that such plaques are not associated with an increased frequency of cerebrovascular events.
When one introduces a new classification, it is important to test the inter-/intraobserver agreement, and we found that the configurations are quite good and reproducible. The overall concordance is good, with a better intraobserver agreement than interobserver agreement. In particular, the type 1 category has excellent values of reproducibility; also, types 4 and 6 have optimal values. Types 2 and 3 have suboptimal values. However, a further evolution to improve the reproducibility could be to apply machine learning algorithms,30 to move from a subjective assessment to a more objective and reproducible type classification.
As expected, we found that the mean degree of stenosis was 64% in symptomatic patients and 35% in asymptomatic ones by confirming that the degree of stenosis is an important parameter, even if this could be considered an indirect parameter influenced by the volume of plaque and the different remodeling pathways.
We have also checked the impact by side, by analyzing the association between the right/left carotid artery plaque configurations and the presence of symptoms (right stroke/left stroke/absence of stroke for asymptomatic patients), and a statistically significant difference for different types of configurations for both right and left plaques was found. Figure 3 shows the relationship between the different plaque configurations on the right/left sides versus the measured risk factors. Moreover, we found that the absence of calcification on one side is not associated with the absence of calcification on the contralateral side: The Cohen κ value was poor (0.297).
To date, there are no grading systems describing carotid artery calcium configurations and distributions. However, there are studies that have demonstrated how some types of configurations can influence the risk of ipsilateral cerebrovascular events. Eisenmenger et al18 showed that the positive rim sign is predictive of an increased stroke risk because it is associated with the presence of IPH within the plaque. Recently, Yang et al26 demonstrated that superficial calcifications were associated with an increased risk of IPH and subsequent risk of cerebrovascular events. Xu et al31 reported a similar association in 2010 using MR imaging. This could be explained by exploring the pathophysiology of the IPH, in which the leakage from the vasa vasorum could produce a blood collection with subsequent space occupation and shift and compression of the adjacent structures with superimposition of the calcification. However, the problem could be also more complex: Van den Bouwhuijsen et al,32 in 2015, found that a larger carotid calcification volume at the level of the bifurcation was related to the presence of IPH. This is an interesting finding because this association did not seem to be driven simply by plaque size or atherosclerotic burden because it did not alter after an adjustment was made for wall thickness. The association was even more pronounced in persons with a low degree of carotid stenosis. They also found that larger calcification volumes were associated with a lower prevalence of lipid core, which was more pronounced at a higher degree of stenosis (>30%).
The findings of this study do not suggest that some types of calcifications are vulnerable but that these are associated with other parameters related to the vulnerability. This concept is clear by comparing types 4 and 6 in which the calcium morphology is exactly the same, but the difference is due to the thickness of the soft-tissue component (<2 and >2 mm for types 4 and 6, respectively). Our results confirm that the role of calcium is complex. It is implicated in different metabolic processes, which are sometimes conflicting and overlapping.17 One important point is that with CT, we can explore a partial element of this complexity, the geometric configuration, and we have found that this could potentially represent a biomarker.
The potential utility of this novel classification relies on the opportunity to classify carotid artery plaque according to the calcium configuration and characteristics, and CT is an optimal technique for this task because it provides excellent visualization of calcification due to the marked x-ray attenuation properties of calcium.33 Further prospective studies would be necessary to test the potential impact of these configurations on the occurrence of cerebrovascular events.34
Our study has limitations. The first one is that due to the retrospective nature of our analysis, the 2 cohorts we have analyzed were not similar due to the statistically significant difference in the hyperlipidemia prevalence. This could have introduced a bias because hyperlipidemia could play a role in the atherogenic process by stimulating some specific pathways. In particular, medial calcification is not associated with atherosclerosis, and it is not typically linked to dyslipidemia, whereas this association is more robust for intimal calcifications35 because hyperlipidemia leads to accumulation of lipoproteins in the subendothelial space of cardiovascular tissues, which leads to formation of mildly oxidized phospholipids, which are known bioactive factors in vascular cell calcification.36,37 Second, this is a retrospective cross-sectional study in which we have not explored the relationship between the types of carotid plaque configurations and the occurrence of cerebrovascular events but we have assessed the relationship between the calcium configuration and the presence of events.
Our data need confirmation in a prospective, longitudinal cohort to confirm our hypothesis and validate our classification; thus, our results should be considered preliminary. Therefore, although suggesting that some types of calcium configurations within the plaque can be markers of instability, we cannot distinguish whether there is a causative relationship between the 2 or if the calcification is a mere epiphenomenon of the concomitant atherosclerotic process. Nevertheless, our results suggest that imaging can explore the complexity of calcium configurations and offer an option of stratification and that calcium configurations may be of value as an indirect marker of plaque instability.
CONCLUSIONS
We suggest a carotid artery plaque configuration classification that is associated with the presence of cerebrovascular events. If confirmed in longitudinal analysis, this classification could be used to stratify the risk of occurrence of ischemic events.
Footnotes
Disclosure forms provided by the authors are available with the full text and PDF of this article at www.ajnr.org.
References
- Received July 14, 2021.
- Accepted after revision November 15, 2021.
- © 2022 by American Journal of Neuroradiology