Abstract
BACKGROUND AND PURPOSE: The treatment of symptomatic carotid near-occlusion is controversial. Our aim was to analyze the results of carotid endarterectomy and carotid artery stent placement in patients with symptomatic carotid near-occlusion and to identify factors related to technical failure, periprocedural complications, and restenosis.
MATERIALS AND METHODS: We conducted a multicenter, prospective nonrandomized study. Patients with angiography-confirmed carotid near-occlusion were included. We assessed the revascularization rate and periprocedural stroke or death. Twenty-four-month clinical and carotid imaging follow-up was performed, and rates of carotid restenosis or occlusion, ipsilateral stroke, and mortality were analyzed. Carotid artery stent placement, carotid endarterectomy, and medical treatment were compared.
RESULTS: One hundred forty-one patients were included. Forty-four carotid artery stent placement and 23 carotid endarterectomy procedures were performed within 6 months after the event. Complete revascularization was achieved in 83.6%, 81.8% in the carotid artery stent placement group and 87% with carotid endarterectomy (P = .360). Periprocedural stroke or death occurred in 6% (carotid artery stent placement = 2.3%; carotid endarterectomy = 13%; P = .077) and was not related to revascularization failure. The carotid restenosis or occlusion rate was 8.3% (5% restenosis, 3.3% occlusion); with carotid artery stent placement it was 10.5%; and with carotid endarterectomy it was 4.5% (P = .419). The 24-month cumulative rate of ipsilateral stroke was 4.8% in the carotid artery stent placement group, 17.4% for carotid endarterectomy, and 13.1% for medical treatment (P = .223). Mortality was 12%, 4.5%, and 5.6%, respectively (P = .422). Revascularization failure and restenosis occurred more frequently in patients with full collapse compared with patients without full collapse (33.3% versus 5.6%, P = .009; 21.4% versus 2.9%, P = .032, respectively).
CONCLUSIONS: Carotid artery stent placement and carotid endarterectomy are associated with high rates of failure and periprocedural stroke. Carotid near-occlusion with full collapse appears to be associated with an increased risk of technical failure and restenosis. Carotid near-occlusion revascularization does not seem to reduce the risk of stroke at follow-up compared with medical treatment.
ABBREVIATIONS:
- CAS
- carotid artery stent placement
- CEA
- carotid endarterectomy
- CHS
- cerebral hyperperfusion syndrome
- CNO
- carotid near-occlusion
- IQR
- interquartile range
The treatment of symptomatic carotid near-occlusion (CNO) is controversial. Post hoc analyses of the NASCET and the European Carotid Surgery Trial (ECST) conducted in the 1990s revealed that the risk of recurrent stroke could be lower in patients with CNO than in patients with conventional severe carotid stenosis and that patients with CNO might not benefit from revascularization.1-3 On the basis of this evidence, the latest guidelines of the European Society for Vascular Surgery do not recommend revascularization in patients with CNO.4 However, recent studies have reported a higher recurrence rate in patients with CNO, especially when associated with full collapse,5,6 and several meta-analyses have shown the nonsuperiority of best medical treatment over revascularization therapies.7-9
Many case series have shown that both carotid endarterectomy (CEA) and carotid artery stent placement (CAS) are effective and safe in patients with CNO.10-15 However, most of these studies are retrospective single-center series that do not even compare the 2 techniques. To date, only 2 retrospective studies have directly compared CEA and CAS in patients with CNO with similar rates of periprocedural complications and long-term recurrences.16,17 Additionally, recent pooled analyses did not show relevant differences between CEA and CAS in CNO in both periprocedural and long-term follow-up.8,9 Unfortunately, we do not have better quality evidence because the latest clinical trials comparing CAS and CEA in the treatment of symptomatic carotid stenosis did not include patients with CNO.18,19
The objectives of this study were to analyze the results obtained with revascularization and medical treatment in patients with symptomatic CNO, to compare periprocedural and follow-up results of CEA and CAS, and to identify factors related to technical failure, periprocedural complications, and restenosis.
MATERIALS AND METHODS
Study Design and Patient Selection
The CAOS study (in spanish “CAsi Oclusión Síntomatica”) is a nonrandomized, prospective, observational, multicenter registry study conducted in 17 Spanish university hospitals from January 2010 to May 2016. The CAOS study methodology has been described previously.20 The study population comprised adult patients with an angiography-confirmed diagnosis of atherosclerotic CNO and ipsilateral ischemic stroke, TIA, or retinal ischemia in the previous 6 months. This study was supported by the “Stroke Project” initiative of the Spanish Cerebrovascular Diseases Study Group and obtained ethics approval from “Comité de Ética e investigación Clínica Hospital General Universitario Gregorio Marañón,” with ID number of the approval: 122/09. Participantsgave informed consent before taking part in the study.
CNO was diagnosed in all patients using DSA. On the basis of the report by Fox et al,1 diagnosis of ICA near-occlusion was confirmed when the angiographic findings fulfilled at least 2 of the following 4 criteria: 1) delayed cranial arrival of ICA contrast compared with the external carotid artery, 2) intracranial collaterals seen as cross-filling of contralateral vessels or ipsilateral contrast dilution, 3) frank reduced diameter of the ICA compared with the contralateral ICA, and 4) reduced ICA diameter compared with the ipsilateral external carotid artery. The presence or absence of a full collapse was determined by each participating center using DSA and/or CT angiography and defined as a “threadlike” lumen distal to the stenosis.21
The treatment decision (medical therapy versus revascularization) as well as the type of revascularization treatment (CEA or CAS) was based on the criteria and routine practice of each participating center. Patients who underwent revascularization beyond 6 months were included in the medical treatment group, and their follow-up was censored at the time of the intervention.
Follow-up and Outcome Measures
The immediate result of the revascularization procedure was assessed in the CAS group by performing an angiographic control series after carotid stent implantation. In the surgical group, a noninvasive carotid imaging test (usually CT angiography or carotid sonography) was performed within 48 hours of the procedure. The result of the revascularization procedure was classified as follows: 1) complete revascularization if a complete or almost-complete recovery of the distal caliber of ICA was achieved, 2) incomplete revascularization when the distal ICA collapse persisted after the intervention, and 3) carotid occlusion when the procedure resulted in complete occlusion of the treated ICA. Revascularization failure was defined as incomplete revascularization or a complete occlusion occurring immediately after the procedure.
Carotid imaging follow-up of the near-occlusion using carotid sonography, CT angiography, and/or MR angiography was performed at 6, 12, and 24 months. Restenosis (defined as a stenosis of >50% of the treated ICA) and complete ICA occlusion rates at 24-month follow-up were collected. Any periprocedural (ie, within 30 days after the procedure) stroke or death rate and other complications were recorded. A clinical follow-up was performed at 3, 6, 12, and 24 months after the presenting event.
The 24-month ipsilateral ischemic stroke rate (including periprocedural events) and mortality were also assessed. Disabling stroke was defined as a stroke associated with a 3-month mRS score of 3–5.
Statistical Analysis
Statistical analyses were performed using the SPSS Statistics 23.0 software package for Windows (IBM). The results were expressed as proportions for categoric variables and as mean (SD) or medians and interquartile range (IQR) for continuous variables. Categoric variables were compared using the χ2 test or 2-tailed Fisher exact test. Continuous variables were compared using a 2-sample t test or the Mann-Whitney test. Univariate and age- and revascularization procedure–adjusted multivariate logistic regression analyses were performed to determine factors related to revascularization failure, periprocedural stroke, or death and restenosis. The cumulative incidences were calculated using Kaplan-Meier curves. Differences among treatment groups were determined using the log-rank test. P values ≤ .05 were considered statistically significant.
RESULTS
A total of 141 patients with angiography-confirmed CNO were recruited. Most patients were men (120; 85.1%), with a mean age of 68.71 (SD, 9.05) years.
Seventy patients underwent revascularization. In 3 of them, the procedure was performed beyond 6 months, and patients were assigned to the medical treatment group. Therefore, 74 patients were included in the medical treatment group, and 67, in the revascularization group (44 CAS and 23 CEA procedures). No significant differences were observed in the baseline characteristics among CEA, CAS, and medical treatment groups (Table 1).
Baseline characteristics of the patients
The median time from the presenting event to the revascularization was 21 days (IQR, 11–43 days), 22 for CEA and 21 for CAS. Ultra-early treatment (ie, within 24 hours) was performed in 4 patients, and 28 patients (41.8%) were treated in the 15 days following the event. One patient had an ipsilateral retinal infarct while awaiting CEA.
Complete revascularization was achieved in 56 patients (83.6%). Revascularization failure was observed in 11 patients (16.4%), incomplete revascularization occurred in 8 patients, and carotid occlusion during the procedure, in 3. No significant differences in the proportion of complete revascularization or failure were observed between CAS and CEA (Table 2).
Revascularization results, periprocedural complications, carotid imaging follow-up, and clinical follow-up
Six patients (8.9%) had a periprocedural stroke or TIA, all of them ipsilateral to the treated CNO. Three strokes or TIAs occurred in the CAS group (6.8%, 1 disabling stroke and 2 TIAs), and the other 3 cases, in the CEA group (13%, all of them nondisabling strokes). The rate of periprocedural stroke or death was 6% (n = 4) and tended to be higher for CEA (13% versus 2.3%, P = .077). Revascularization failure was not associated with an increased risk of periprocedural events: Only 1 patient treated with CAS had an ICA occlusion and ipsilateral disabling stroke. None of the patients died within the first 30 days following the procedure, though 1 patient treated with CAS who had a cerebral hyperperfusion syndrome (CHS) died 67 days after the procedure. Twelve other periprocedural complications were registered (Table 2). CHS was recorded in 2 cases (3%), with no differences between surgical and endovascular treatments.
Carotid imaging follow-up was performed in 60 patients. Restenosis or ICA occlusion was detected in 5 patients (8.3%). Restenosis occurred in 3 patients (5%), all of them treated with CAS. In 1 patient, restenosis was symptomatic, presenting as an ipsilateral TIA. Progression to complete ICA occlusion was observed in 2 patients (3.3%), one (4.8%) in the surgical group and another (2.3%) in the CAS group (P = .636). The patient who progressed to ICA occlusion after endovascular treatment had a disabling ipsilateral stroke (Table 2).
Revascularization failure occurred more frequently in patients with full collapse: 33.3% compared with 5.6% (P = .009). Additionally, restenosis or ICA occlusion was more frequent in patients with CNO with full collapse (21.4% versus 2.9%, P = .032). No significant relationship was observed between the presence of full collapse and the risk of periprocedural stroke or death (6.7% in CNO with full collapse and 8.3% in CNO without full collapse, P = .840) or with CHS (6.7% and 2.8%, P = .514). In the multivariate analysis, the presence of full collapse was the only factor independently related to an increased risk of revascularization failure (OR, 11.6; 95% CI, 1.6–84.6) and restenosis at follow-up (OR, 27.4; 95% CI, 1.3–567.7) (Table 3).
Factors related to revascularization failure, periprocedural stroke or death, and carotid restenosis
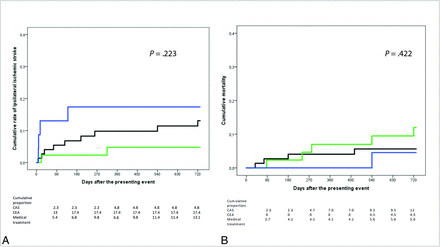
Fifteen patients had an ipsilateral ischemic stroke during the 24-month follow-up, resulting in a cumulative rate of 11.1% (95% CI, 5.8%–16.4%). Two ipsilateral strokes occurred in the CAS group (cumulative rate, 4.8%; 95% CI, 0%–11.3%), 4 in the surgical group (1 before CEA and 3 in the periprocedural period, cumulative rate, 17.4%; 95% CI, 1.9%–32.9%), and 9 in the medical treatment group (13.1%; 95% CI, 5.1%–21.1%; P = .223) (Figure). We recorded 6 disabling strokes: 2 in the endovascular group (cumulative rate, 4.8%), none in the CEA group, and 4 in the medical treatment group (5.8%, P = .518).
Kaplan-Meier curves comparing the effect of CAS (green line), CEA (blue line), and medical treatment (black line) on the cumulative rate of ipsilateral ischemic stroke (A) and mortality (B).
Ten patients died during follow-up. The 24-month cumulative mortality rate was 7.5% (95% CI, 3.0%–12.0%). Five deaths occurred in the endovascular group (12%; 95% CI, 2.2%–21.8%), 1 patient died in the CEA group (4.5%; 95% CI, 0%–13.1%), and 4, in the group of patients treated medically (5.6%; 95% CI, 0.3%–10.9%) (P = .422; Figure).
DISCUSSION
In the present study, a revascularization rate of 84% was obtained with no significant differences between CEA and CAS. Our revascularization results are lower than those observed in previous series with success rates well above 90%.10,11,15-17 However, these results come from retrospective single-center studies in which selection biases cannot be ruled out.
Traditionally, CNO revascularization has been associated with a high risk of complications. However, the NASCET and ECST studies showed similar periprocedural stroke and death rates for CNO and conventional ICA stenosis (5.4% and 6.2%, respectively),2 and a recent meta-analysis found a perioperative risk of 4.8% for CEA and 5.4% for CAS.8 The periprocedural stroke and death rate was 6% in our study and was especially high in the CEA group (13% versus 2.3%). These poor results should be viewed with caution considering the small number of patients included in each treatment group, which could be overstating some of our results. Furthermore, all 3 perioperative strokes that occurred in the CEA group were nondisabling.
The incidence of CHS after carotid revascularization ranges between 0% and 3%.22 However, a recent meta-analysis described a higher incidence for patients treated with CAS, reaching 4.6%.23 The incidence of CHS could also be higher in patients with CNO: Cay et al24 reported an 8.6% rate after CAS and observed a significantly higher risk among patients with full collapse (30% compared with 4.2% in CNO without full collapse). A decreased cerebral autoregulation or underlying blood-brain barrier damage has been proposed to explain this finding. In our study, CHS occurred in 3% of cases, and no differences were observed between CAS and CEA or between CNO with or without full collapse.
Restenosis or ICA occlusion at 2-year follow-up was detected in 8.3% of our patients and occurred more frequently with CAS (10.5% versus 4.5%), though the difference did not reach statistical significance. Pooled analyses found a rate of restenosis in patients with CNO that ranged between 4% and 6.4% for CEA and 4.1% and 7.5% for CAS,8,9 not very different from the restenosis rate of 4%–5% at 1 year for conventional ICA stenosis described in the Carotid Revascularization Endarterectomy Versus Stenting Trial (CREST).18 However, Kim et al17 observed a higher proportion of restenosis in CNO, reaching 20% for CAS and 17.4% for CEA.
Recent meta-analyses have described a long-term stroke rate below 5% in patients with CNO who undergo revascularization.8,9 The 24-month cumulative stroke rate is strikingly higher in our study, especially for CEA, reaching 17%. These results are clearly related to the high rate of periprocedural stroke observed in proportion to the small number of patients included in this treatment group. On the other hand, if we re-analyze the cumulative recurrence rate in the CEA group and consider the event that occurred before surgery as a recurrence in the medical treatment group, the cumulative rates of both groups would be similar (13.6% for CEA and 14.3% for medical treatment). In any case, our study has not been able to show clear differences in the risk of recurrent stroke at 2 years among the different treatment groups.
Full collapse has been associated with an increased risk of recurrence in medically treated patients with CNO,5,6 though this effect has not been confirmed in all studies.25
On the other hand, the impact of full collapse on the results of the revascularization of CNO has been less often studied. In our study, CNO with full collapse was associated with a greater probability of revascularization failure (33% versus 6%). This finding may be explained by the technical difficulties involved in fully expanding the stent in a narrow artery or in implanting a shunt in patients treated with CEA. The study by Neves et al26 also showed a high failure rate with CAS (21%) in patients with CNO and full collapse. Other studies do not seem to confirm these findings.24,27 We also observed an increased risk of restenosis or ICA occlusion in patients with collapsed CNO. Meershoek et al27 did not record restenosis in their 17 patients with CNO with full collapse treated with CEA, and Neves et al described 2 occlusions during follow-up in their 19 patients treated with CAS. Recently, Johansson et al28 have described a high rate of postprocedural stroke or death in patients with CNO and full collapse (2 of 10 versus 0 of 43 in CNO without full collapse and 5 of 166 in conventional carotid stenosis). Most interesting, the 2 cases described were intracerebral hemorrhages. In our study, no association was observed between the presence of full collapse and the risk of periprocedural stroke or death.
This study has some limitations. First, this is a subanalysis of a previous study, which was not specifically designed to compare CEA, CAS, and medical treatment. Second, the sample size of the study does not have sufficient statistical power for an adequate comparison between CEA and CAS; therefore, the periprocedural stroke rate in the CEA group could have been overestimated. On the other hand, the small number of patients with CNO and full collapse may have affected the precision of the multivariate analysis. Third, patients were not randomized to medical or revascularization treatment and CEA or CAS; treatment decisions were based on the criteria of each participating center. Fourth, the study provides limited information on the technical aspects of the different treatments used. The main strength of this study is its prospective design and the use of predefined diagnostic criteria for CNO.
CONCLUSIONS
Our results suggest that revascularization of CNO is a technically challenging procedure with a high failure rate and an increased risk of periprocedural stroke and other potentially serious complications such as CHS. In addition, revascularization in patients with CNO with full collapse appears to be associated with a higher risk of technical failure and restenosis. On the other hand, CAS or CEA was not associated with a significant reduction of ipsilateral stroke at follow-up compared with medical treatment.
These findings raise new questions about the optimal management of CNO and force us to consider the risk-benefit balance between medical treatment and revascularization in this group of patients.
Future research in CNO should focus on the following:
A better understanding of the pathophysiology and natural history of CNO
Elucidating the impact that the presence or absence of full collapse may have in patients with CNO
Identifying other factors associated with an increased risk of stroke
Studies specifically designed to allow comparison between medical treatment and revascularization and between CEA and CAS.
Footnotes
Disclosure forms provided by the authors are available with the full text and PDF of this article at www.ajnr.org.
References
- Received March 15, 2022.
- Accepted after revision June 17, 2022.
- © 2022 by American Journal of Neuroradiology