Abstract
BACKGROUND AND PURPOSE: Outward convexity of the basiocciput and posterior atlanto-occipital membrane are common in patients with Chiari II malformation associated with an open neural tube defect. We aimed to determine if the severity of these findings correlated with the need for future hydrocephalus treatment.
MATERIALS AND METHODS: A retrospective chart and imaging review identified patients who underwent open neural tube defect repair at a quaternary care pediatric hospital from July 2014 through September 2022. Patients were classified by the need for hydrocephalus treatment and whether they received prenatal or postnatal neural tube defect repair. Measurements of imaging parameters related to posterior fossa maldevelopment and skull base remodeling were performed.
RESULTS: Compared with 65 patients who did not require hydrocephalus treatment, 74 patients who required treatment demonstrated statistically significantly greater mean basiocciput convexity (P < .001). While the mean basiocciput length in the hydrocephalus treatment group was smaller (P < .001), the ratio of basiocciput convexity to length was larger (P < .001). Notably, 100% of patients with a basiocciput convexity of ≥4 mm required hydrocephalus treatment. The mean posterior atlanto-occipital membrane convexity was significantly greater for patients who required hydrocephalus treatment in the postnatal group (P = .02), but not the prenatal group (P = .09).
CONCLUSIONS: Pediatric patients with Chiari II malformation who ultimately required surgical hydrocephalus treatment had greater outward convexity of the basiocciput but had greater posterior atlanto-occipital membrane outward convexity only if the repair was performed postnatally. Together these measurements may be useful in predicting the need for hydrocephalus treatment.
ABBREVIATIONS:
- CM2
- Chiari II malformation
- FOD
- frontal occipital diameter
- HT
- hydrocephalus treatment
- MMC
- myelomeningocele
- ONTD
- open neural tube defect
- PAOM
- posterior atlanto-occipital membrane
- PFD
- posterior fossa diameter
Neural tube defects remain common, occurring at an approximate prevalence of 5–7 per 10,000 live births.1 Neural tube defects have been classified as closed (skin-covered) or open (nonskin-covered), with open neural tube defects (ONTDs) including myelomeningocele (MMC) and myelocele (also known as myeloschisis).2 ONTDs are typically associated with Chiari II malformation (CM2), and this relationship has been explained by the unified theory of McLone and Knepper,3 which suggests that the leakage of CSF into the amniotic fluid through the defect leads to inadequate expansion of the posterior fossa embryonal vesicles and consequently characteristic findings of CM2.4 On the basis of this current understanding, fetal surgery with intrauterine repair of the ONTD is performed to reduce the degree of CM2 and possibly the postoperative necessity for ventriculoperitoneal shunting.
Hydrocephalus is a common complication of CM2 and frequently requires surgical intervention. ONTDs and CM2 are associated with significant morbidity and mortality,5 and hydrocephalus seen with CM2 is a significant contributor. Surgical hydrocephalus treatment (HT) has traditionally encompassed ventriculoperitoneal shunting, the complications of which can also contribute to poor outcomes. More recently, endoscopic third ventriculostomy and choroid plexus cauterization are increasingly being used in HT as an alternate strategy to ventricular shunting.6
The pathophysiologic mechanisms leading to hydrocephalus in CM2 are complex and incompletely understood, making predicting which patients will need surgical HT difficult. Identifying patients at greatest risk for future hydrocephalus development and the need for HT may help in clinical management and counseling. Previous studies have identified multiple characteristics correlated with the need for future HT. The more cephalad the level of the MMC defect, the higher will be the incidence of ventricular shunting.7 For patients who underwent prenatal repair of ONTDs, smaller ventricle size and defects located at or below L4 were less likely to require shunting.8 The Management of Myelomeningocele Study (MOMS) investigators, in a report of the 1-year outcomes from the MOMS trial, demonstrated that larger ventricle size on imaging before prenatal repair was associated with an increased need for future shunting.9 Zarutskie et al10 demonstrated the predictive value of multiple imaging parameters with the need for HT in patients who underwent prenatal MMC repair, with the best predictors being presurgery ventricular width, persistent hindbrain herniation postrepair, and growth in ventricular volume.
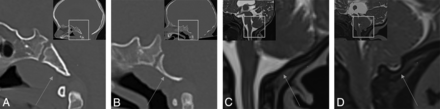
However, the prognostic value of imaging parameters related to posterior fossa maldevelopment and skull base remodeling in CM2 and their correlation with the need for future HT remain unreported, despite their possible contributing component in hydrocephalus development. Several osseous skull base changes occur with posterior fossa maldevelopment in patients with CM2, including reduced posterior fossa volume, reduced supraocciput and exocciput lengths,11 a small dorsum sellae,12 petrous scalloping,13 as well as hypoplasia and altered morphology of the occipital bone. The severity of scalloping, or outward convexity of the clivus, was found to be significantly greater in patients with MMC and CM2,11 and in our experience, it is a frequently seen finding. Additionally, outward convexity of the posterior atlanto-occipital membrane (PAOM) is frequently seen in CM2 (Fig 1), though it is of uncertain prognostic significance. The goal of our study was to determine if outward convexity of the basiocciput and PAOM are associated with the need for future HT, which may provide prognostic value as well as possible insight into the mechanisms of hydrocephalus development.
Comparison of normal and remodeled basiocciput and posterior atlanto-occipital membranes. A and B, Sagittal CT images demonstrate the normal appearance of the basiocciput (arrow in A) and outward convexity of the basiocciput (arrow in B). C and D, Sagittal T2-weighted images demonstrate the normal appearance of the PAOM (arrow in C) and outward convexity of the PAOM (arrow in D). Image insets demonstrate larger FOV sagittal images and the location of corresponding zoomed-in FOV.
MATERIALS AND METHODS
The study was approved by the Baylor College of Medicine institutional review board. A retrospective chart and imaging review was performed on initial brain CT and/or MR imaging studies obtained among patients diagnosed with ONTD (MMC or myelocele) who underwent either in utero or postnatal repair from July 2014 through September 2022 at a large quaternary care pediatric hospital with an integrated dedicated fetal treatment center. Patients were identified from a surgical database and were included if a postnatal head CT or brain MR imaging was performed. Patients were grouped according to those that required neurosurgical HT and those who did not and were additionally stratified according to whether they received prenatal or postnatal surgical repair of their ONTD. The presence or absence of HT was determined by chart review assessing a history of prior ventriculoperitoneal shunting or endoscopic third ventriculostomy/choroid plexus cauterization. For the group that did not require HT, patients were excluded if <12 months of clinical follow-up was available. Additionally, patients were excluded from the analysis if no head CT or MR imaging was available for review before HT, if the performed imaging was nondiagnostic, as well as if they demonstrated severe cerebellar parenchymal volume loss or had a known history of an acute or chronic CNS injury other than the neural tube defect (eg, history of intraventricular hemorrhage or meningitis).
Radiology assessment was performed by a neuroradiologist with additional fellowship training in pediatric neuroradiology. All measurements were performed on the initial/earliest postnatal CT or MR imaging study and before HT. MR imaging sequences were chosen on the basis of the best subjective evaluation of the posterior fossa structures within the limitation of motion and artifacts, and high-resolution fluid-sensitive MR imaging sequences were used when available. Measurements were obtained before knowledge of the future shunt status.
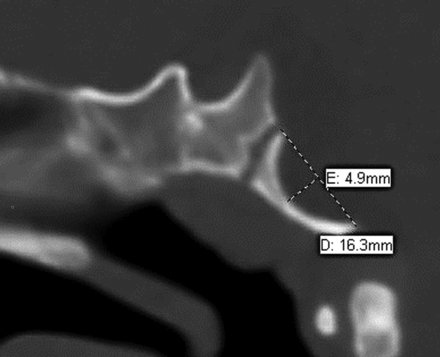
The basiocciput length was measured by placing a line along the dorsal aspect of the basiocciput from the basion to the dorsal spheno-occipital synchondrosis. The severity of basiocciput outward convexity was then measured as the maximum distance to the dorsal basiocciput orthogonal to the basiocciput length line (Fig 2). Because the convexity predominately occurred within the basiocciput with relative sparing of the basisphenoid, the measurements were limited to the convexity of the basiocciput. Distance, rather than angle,11 was used for reproducibility and simplicity. To control for differences in the total size of the basiocciput affecting the convexity measurements, we compared the ratio of the basiocciput convexity with the basiocciput length.
Sagittal CT of the skull base demonstrating outward convexity of the basiocciput in a 10-month-old infant with CM2. Basiocciput length is measured from the basion to the dorsal spheno-occipital synchondrosis. Convexity is measured as the maximum distance orthogonal to the length line.
The severity of the PAOM outward convexity was measured in a similar fashion. First, the C1 opisthion line was drawn from the superior aspect of the posterior C1 arch to the superior aspect of the opisthion. Then, the largest distance from the convexity of the PAOM orthogonal to the distance line was measured (Fig 3).
Sagittal T2-weighted image of the posterior craniocervical junction in a 1-month-old infant with CM2. C1-opisthion length is measured from the posterior C1 arch to the opisthion. Posterior atlanto-occipital membrane convexity is measured as the maximum distance orthogonal to the length line.
To estimate posterior fossa size, we measured the posterior fossa diameter (PFD) using the Twining line drawn between the dorsum sellae and the internal occipital protuberance.14 The PFD was then compared with the frontal occipital diameter (FOD), measured as the largest dimension between the frontal and occipital calvaria in the midsagittal plane.15 The ratio of these measurements was then calculated (FOD/PFD) to estimate the relative posterior fossa size, using linear measurements for simplicity and ease of measurement.15,16
To assess the severity of ventriculomegaly, we measured the ventricular width with a line drawn at the maximum width of the atria of the lateral ventricles in the coronal plane, using the widely accepted method described for measurement of fetal ventriculomegaly.17
Statistical comparisons between the 2 groups were performed using an unpaired Student t test with GraphPad Prism software (Version 9.4.1, GraphPad Software) and Excel Microsoft (Office Professional Plus 2016). Values of P < .05 were considered statistically significant.
RESULTS
A total of 318 patients underwent surgical ONTD repair; 168 patients had an available head CT or brain MR imaging and were included in the study. Of these patients, there were 29 total patients excluded from the study: 17 were excluded for lack of a head CT or brain MR imaging before HT, and 12 were excluded for nondiagnostic imaging or if they demonstrated severe cerebellar parenchymal volume loss or had a known history of an acute or chronic CNS injury other than the neural tube defect. A total of 139 patients were identified for study analysis, of which 70 patients underwent prenatal ONTD repair and 69 patients underwent postnatal repair. Ultimately, 74 of 139 patients required HT. The mean gestational age at birth was 37.1 (SD, 2.8) weeks for patients who required HT and 37.2 (SD, 3.2) weeks for patients who did not require HT (P = .82). The mean age at the time of the first head CT or MR imaging was 3.2 (SD , 3.9) months (minimum = 1 month, maximum = 20 months) in patients who required HT, and 12.1 (SD, 8.3) months (minimum = 1 month, maximum = 35 months) for patients who did not require HT (P < .001). For patients who required HT, the mean age at the time of HT was 4.7 (SD, 6.9) months (minimum = 1 month, maximum = 44 months). For patients who did not require HT, the mean clinical follow-up was 39.5 (SD, 22) months (minimum = 12 months, maximum = 103 months).
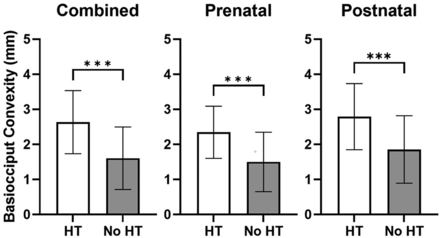
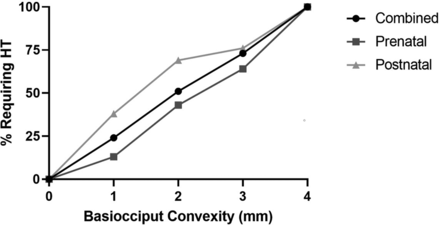
The mean basiocciput outward convexity on the first diagnostic CT or MR imaging was significantly greater in patients who ultimately received HT compared with patients in whom HT was not required in both the prenatal (2.3 mm; range, 1–4 mm compared with 1.5 mm; range, 0–3 mm; P < .001) and postnatal (2.8 mm; range, 1–4 mm compared with 1.9 mm; range, 0–3 mm; P < .001) repair groups, as well as both groups combined (2.6 mm; range, 1–4 mm compared with 1.6 mm; range, 0–3; P < .001) (Fig 4). The percentage of patients requiring HT increased with increasing severity of the basiocciput convexity. Notably, 100% of patients with basiocciput convexity of ≥4 mm required HT in our cohort (Fig 5).
Basiocciput convexity is increased in patients requiring HT. Basiocciput convexity (millimeters) was measured in all patients requiring HT and not requiring HT. Patients were then stratified by prenatal or postnatal repair of the ONTD and basiocciput convexities and were compared. Bars represent the mean (SD) with triple asterisks P < .001 representing statistical significance as determined by an unpaired Student t test.
An increase in basiocciput convexity was associated with a higher percentage of patients requiring HT. The percentage of patients requiring HT at varying basiocciput convexities (1–4+ mm) was determined in the combined prenatal and postnatal cohorts.
Compared with patients who did not require HT, patients who required HT demonstrated significantly greater mean basiocciput convexity-to-length ratio (P < .001) and FOD/PFD ratio (P < .001), as well as significantly less mean basiocciput length (prenatal, P < .001; postnatal, P = .002), and mean PFD (P < .001) (Table 1).
CT and MR imaging data of patients who underwent either prenatal or postnatal surgical repair of an ONTD, according to whether they required HTa
The mean PAOM convexity was significantly greater for patients who required HT in the postnatal group (P = .02), but not the prenatal group (P = .09). The mean lateral ventricle width (average width of the right and left lateral ventricles for each patient) was significantly greater for patients who required HT in the prenatal group (P = .02), but not in the postnatal group (P = .54).
When we compared the prenatal and postnatal repair groups without consideration of the need for HT, there were significant differences between groups (Table 2). Compared with the postnatal repair group, the prenatal repair group demonstrated significantly smaller mean basiocciput convexity and mean basiocciput convexity/length ratio. There was no significant difference in the mean convexity of the PAOM between the prenatal and postnatal repair groups.
CT and MR imaging data of all patients regardless of whether they received HT, according to whether they received prenatal or postnatal surgical repair of an ONTDa
DISCUSSION
Previous studies have evaluated the correlation of several imaging characteristics with the need for future HT in patients with CM2 associated with ONTDs.7⇓⇓-10 To our knowledge, correlation between imaging characteristics related to posterior fossa maldevelopment, specifically the frequently seen outward convexity of the basiocciput and the need for future HT, has not yet been reported. Our findings indicate that the severity of basiocciput convexity is significantly associated with the future need for HT in both prenatal and postnatal repair groups. We found that 100% of patients required HT with a basiocciput convexity of ≥4 mm on initial head CT or MR imaging performed in the first 3 years of life, suggesting possible use of this assessment in clinical practice.
The mechanism for the development of this outward convexity is unclear. The lengths of the basiocciput were smaller in patients requiring HT in both prenatal and postnatal repair groups, suggesting more significant hypoplasia of the basiocciput in the HT group. This suggestion is consistent with our finding that the posterior fossa diameter is also smaller in the HT group and is consistent with the known occurrence of occipital bone hypoplasia in the setting of CM2.11 However, our findings indicate that when we controlled for differences in the length of the basiocciput using the basiocciput convexity-to-length ratio, the convexity was still significantly correlated with the need for HT, possibly implying a mechanism unrelated to occipital bone hypoplasia. One possible explanation for the outward convexity is that as the CSF pressure increases in the setting of worsening hydrocephalus, there is pressure-induced outward remodeling of the basiocciput. However, the unified theory suggests that CSF leakage through the ONTD leads to low CSF pressure, which subsequently leads to posterior fossa maldevelopment.3 Therefore, though not directly assessed in the present study, documentation of consistent basiocciput outward convexity before ONTD repair would suggest that just abnormal CSF pressure is unlikely to be the cause.
Another possible mechanism could be mass effect related to accommodation of the posterior fossa contents in the setting of a small posterior fossa in CM2. The proximity of the belly of the pons to the dorsal basiocciput in the setting of hindbrain herniation before ONTD repair may be responsible for the outward convexity of the basiocciput. Interestingly, there were some cases noted during this retrospective review in which the hindbrain herniation was so extreme that the pons descended to the level of the foramen magnum; in these cases, the foramen magnum appeared expanded and there was notably less basiocciput curvature. This feature would provide support for the mass effect from the pons as a primary mechanism. If true, the degree of basiocciput convexity may function as a surrogate and quantitative measure for more severe initial hindbrain herniation before repair, but not for extreme hindbrain herniation. Further studies may help to establish a causative mechanism, explain the correlation with future HT, and provide insight into the pathophysiology of hydrocephalus.
While the outward convexity of the PAOM was significantly correlated with the need for HT in the postnatal repair group, it was not significantly correlated in the prenatal repair group, though there was a trend toward significance (P = .08). A possible cause of the lack of correlation in the evaluation of the PAOM convexity is differences in flexion/extension positioning of the head, because flexion of the head relative to the cervical spine causes a lengthening of the PAOM and may result in a reduction of the measurable outward convexity. Additionally, while a larger mean ventricle size was significantly correlated with the need for HT in the prenatal repair group, it was not significantly correlated in the postnatal group.
Study limitations include the retrospective nature, image evaluation performed by a single radiologist, lack of head CT or MR imaging studies in patients without ventriculomegaly or clinical signs or symptoms or hydrocephalus (instead, head ultrasound and clinical follow-up were used), differences in age at the time of initial head CT or MR imaging between patients who required HT and those who did not (which was due to the differences in clinical need for imaging), and variable duration of follow-up to assess HT. Measurements were performed on either CT or MR imaging, possibly introducing measurement error in comparing the dimensions of an osseous structure, which is better evaluated by CT; further studies may be helpful to assess significant differences in measurements between modalities. In addition, our study does not evaluate absolute intracranial pressure measurements and does not evaluate altered CSF flow pulsatility, which may play a role in the skull base remodeling. The main study strengths include the large sample size as well as all patient management occurring at a single institution, possibly reducing variability in the decision to perform neurosurgical HT.
CONCLUSIONS
In pediatric patients with CM2 related to an ONTD, the severity of the basiocciput outward convexity was significantly correlated with the need for future neurosurgical HT in both prenatal and postnatal ONTD repair groups. The convexity of the posterior atlanto-occipital membrane was significantly correlated with the need for postnatal hydrocephalus treatment in patients with postnatal but not prenatal spinal neural tube defect repairs. Notably, 100% of patients required HT with a basiocciput convexity of ≥4 mm. These measurements may be useful in predicting the need for HT.
Footnotes
Disclosure forms provided by the authors are available with the full text and PDF of this article at www.ajnr.org.
References
- Received October 8, 2023.
- Accepted after revision December 11, 2023.
- © 2024 by American Journal of Neuroradiology