Abstract
BACKGROUND AND PURPOSE: Traditionally cerebellar functions are thought to be related to control of tone, posture, gait, and coordination of skilled motor activity. However, there is an increasing body of evidence implicating the cerebellum in cognition, language, memory, and motor learning. Preterm infants are at increased risk of neurodevelopmental delay, cognitive dysfunction, and behavioral and emotional disturbances. The role of the cerebellum in these adverse outcomes is unclear.
Objective:The objective of this study was to determine whether absolute cerebellar volumes differ between term-equivalent preterm infants and term-born control infants and to assess whether cerebellar volume is influenced by any possible antenatal, perinatal, and postnatal factors.
METHODS:The study compared the MR imaging cerebellar volume by using a manual quantification program of 113 preterm infants at term-equivalent age and 15 term-born control infants.
RESULTS:The median cerebellar volume of preterm at term-equivalent age was 25.4 cm3 and that of term-born control infants was 26.9 cm3. On initial analysis, there was a significant median difference of 2.0 cm3 (95% CI, 1.2 cm3 to 2.7 cm3) (2-sided P < .0001). However multiple regression analysis of perinatal variables showed that only infants with supratentorial lesions (P = .003) were significantly associated with the reduction in cerebellar volumes. The median cerebellar volumes were the following: supratentorial lesions, 18.9 cm3; no supratentorial lesions, 26.1 cm3; and term infants, 26.9 cm3 (analysis of variance, P < .0001). Hence, there was no significant difference in cerebellar volumes of preterm infants at term-equivalent age in the absence of supratentorial lesions. The median vermal volumes were 0.7 cm3 and were significantly related to cerebellar volumes both in preterm infants with and without lesions and in term-control infants.
CONCLUSION:Premature infants at term-equivalent age have similar total cerebellar and vermal volumes compared with term infants in the presence of normal brain imaging. Reduced cerebellar volume in preterm infants at term-equivalent age is seen in association with supratentorial pathology such as hemorrhagic parenchymal infarction, intraventricular hemorrhage with dilation, and periventricular leukomalacia.
Modern neonatal and perinatal care has lead to continual improvement in the survival rates of extremely preterm infants.1 Although the survival rate has improved to 44% for infants born at 25-weeks gestation and to >85% for infants weighing <1000 g,1,2 there is growing concern about the 25%–50% neurodevelopmental delay and 30%–37% cognitive delay3-5 that these infants have in later life. Even preterm infants without a complicated perinatal course may have serious cognitive problems.6 Children born preterm (<30-weeks gestation) have an IQ that is 1 standard deviation (SD) below the population mean, and more than half of these children need special assistance in school.7,8 These children also have emotional and language disturbances,7,9-12 and approximately 22% have at least 1 psychiatric disorder such as attention deficit hyperactivity disorder, disruptive disorder, or obsessive-compulsive disorder.8
In addition to these cognitive abnormalities, preterm infants also manifest a constellation of subtle neurologic signs, including dysdiadochokinesia, poor coordination of fine movements, and impaired motor sequencing, which has been termed “developmental coordination disorder”13 and is thought to be related to cerebellar abnormalities.
In the past decade, the cerebellum has been implicated in higher functions such as cognition, motor learning, language, and memory,14-16 based on anatomic, functional neuroimaging, physiologic, pathologic, and postmortem studies, in addition to traditional functions such as posture, gait, and control of tone and coordination of skilled motor activity.15,17 Hence, the role of cerebellum in the neurocognitive abnormalities of preterm infants is being investigated.
Previous studies have shown that the size of the cerebellum in preterm infants at 8–15 years of age is significantly reduced compared with that of control children.18,19 Allin et al18 showed that the cerebellar volume was reduced by 12% and that this reduction was significantly associated with several cognitive tests. However, these infants did not have early MR imaging, and preterm infants with and without lesions were combined in their analysis. Therefore it remains unclear when and why this poor cerebellar growth occurs and whether the reduction in cerebellar volume is seen in all preterm infants.
Limperopoulos et al20 have shown that the rate of cerebellar growth is similar in preterm infants with and without supratentorial lesions. They also found that the smaller cerebellar volume was significantly related to lower gestational age. However, the number of preterm infants in their study who had paired follow-up scans at term-equivalent age was biased toward infants with lesions. Hence, it is not known whether impaired cerebellar growth is present in the absence of supratentorial lesions.
The aim of this study was to determine the absolute cerebellar volumes of term-equivalent preterm infants and term-born control infants. We hypothesized that cerebellar volume would be reduced in preterm infants at term-equivalent age. Further exploratory analysis was performed to assess whether cerebellar volume is influenced by the presence of antenatal, perinatal, and postnatal factors or by the presence of cerebral lesions.
Methods
Subjects
This study was approved by the Trust Research Ethics Committee of Hammersmith Hospital and informed parental consent was obtained. The cases included preterm infants born at <34 weeks gestation who underwent MR volume-acquisition imaging at term-equivalent age from 2000 to 2003. Infants with congenital malformations, metabolic disease, pre-existing lesions diagnosed antenatally, congenital infections, and obvious cerebellar anomalies on MR imaging were excluded. Infants in the control group were recruited from the postnatal ward.
Patient Characteristics
All the infants had standard neonatal care at the Hammersmith Hospital neonatal unit. The data on antenatal, perinatal, and postnatal factors, including some potential confounding factors, have been collected from the patients’ notes and from 2 pre-existing data bases (clinical and research). This included data about maternal age, smoking, prolonged rupture of membranes, infection, gestational age (calculated from last menstrual period and/or early sonography scans), multiple pregnancy, sex, birth weight, intrauterine growth restriction (birth weight, <10th percentile intrauterine growth retardation [IUGR]), head circumference, antenatal and postnatal steroids, number of days on a ventilator, bronchopulmonary dysplasia (diagnosed if the infant was requiring supplemental oxygen at 28 days with abnormal chest X-ray findings), chronic lung disease (if the infant was needing supplemental oxygen at 36 weeks postconceptional age), inotropic support, patent ductus arteriosus, days on total parenteral nutrition, time to feed, septic episodes, necrotizing enterocolitis, and supratentorial lesions (intraventricular hemorrhage with dilation and hemorrhagic parenchymal infarction and periventricular leukomalacia.)
MR Imaging
All images were obtained with a 1.5T magnet (Philips Medical Systems, Best, the Netherlands). A trained pediatrician was present during scanning. Preterm infants were sedated with chloral hydrate for scanning. Term-born controls were fed and wrapped, and scanning was performed in natural sleep. All the infants had conventional T1-weighted and T2-weighted fast spin-echo imaging in the transverse plane. T1-weighted 3D radio-frequency spoiled volume images were obtained in the sagittal plane (TR/TE, 30/4.6 ms; flip angle, 30°; field of view, 25 cm; 156 × 256 matrix; and 1 excitation). One hundred fourteen sections were taken for each patient. The section thickness was 1.6 mm, and the in-plane resolution was 0.98 × 0.98 mm. Images were assessed for normal appearance of the cerebellum and for supratentorial lesions such as periventricular leukomalacia, hemorrhagic parenchymal infarction, and ventricular dilation with or without residual intraventricular hemorrhage.
Cerebellar Quantification
The volume of the cerebellum was quantified by using the T1-weighted volume images. Manual outlining of cerebellar tissue was performed for each cerebellar section by using a contour encompassing both the white and gray matter of the cerebellum as shown in Fig 1. The cerebral peduncles have been included in the determination of volume.
The white manual outlining of the cerebellum of one of the T1-weighted volume acquisition scans in the sagittal plane.
The volume of the each section was calculated by multiplying the total voxel area within the contour and the section thickness. The total cerebellar volume was calculated by addition of all cerebellar volume sections. The vermal surface area was assessed by using a contour that gave the maximum cross-sectional area of the vermis.21 The vermal volume was then calculated in a manner similar to that of the whole cerebellar volume. The volumes of both cerebellar hemispheres were assessed individually from the section after the vermis to the lateralmost sections.
Inter- and Intraobserver Correlation
The interobserver and intraobserver correlation of assessments was calculated from repeating measurements on 20 scans by 2 different observers and from repeating the measurements on the 20 scans twice by the same observer respectively.
Power and Precision Calculation
To detect a difference of 2 cm3 between cerebellar volumes of preterm infants at term-equivalent age and term-born controls with 80% power and a P value of < .05, given the SD of 5 cm3, we then calculated the number needed in each arm of study to be 45 infants. Because there were only 15 infants available in the control group, the number needed in the preterm group was calculated to be 118 from the formula for unequal sample sizes. Precision calculation also showed similar numbers to be 95% confident that the difference in the mean of cerebellar volumes between the cases and controls was within ±2.1 cm3 of the true population.
Statistical Analysis
The statistical analysis was performed by using StatsDirect (version 2.0.1 for Windows). The data were tested for normality by the Shapiro-Wilkes test before each analysis, and parametric or nonparametric descriptive statistics and hypothesis testing was used as appropriate. The difference in the median of the cerebellar volume was analyzed by a Mann-Whitney U test. Patient characteristics were analyzed by using t test and χ2 analyses as appropriate. Analysis of variance (ANOVA) and multiple regression were performed for the cerebellar volume by using head circumference at scanning, weight at scanning, and cerebral volume entered as independent variables to control for effects of generalized scaling within the brain. The analysis was also repeated with birth weight and gestational age entered as covariates to control for generalized scaling effects.
In the exploratory analysis, multiple regression analysis was performed. Multiple comparisons of cerebellar volumes were performed by using ANOVA with the Bonferroni multiple comparison tests. The Kendall rank correlation and analysis of agreement were performed for inter- and intrarater agreement.
Results
A total of 118 preterm infants and 15 term controls were recruited. Five infants in the preterm group were excluded because of the poor quality of scans. None of the infants had an abnormal appearance of the cerebellum on visual inspection of the images.
Patient Characteristics
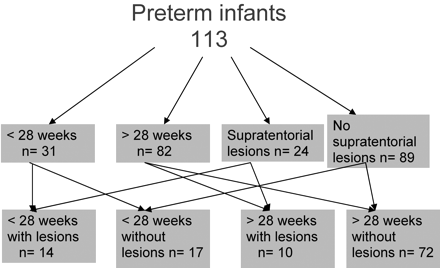
Preterm infants had a median birth weight of 1.09 kg (lower quartile, 0. 69 kg; upper quartile, 1.5 kg) and a median gestational age of 29 weeks (lower quartile, 26.14; weeks; upper quartile, 31.57 weeks). Forty-six percent were boys and 63% were single births. Sixty-two percent of the infants had received antenatal steroids, and 15% had signs of chorioamnionitis. Fifty percent of the infants were ventilated, and 53% received surfactant. Continuous positive pressure ventilation was needed in 67% of the infants. Figure 2 summarizes the gestational age groups and pathologic characteristics of preterm infants at term-equivalent age.
Description of various subgroups in preterm infants at term-equivalent age.
Fifteen term infants were recruited from the postnatal ward. Nine of these infants were boys. The mean gestational age at birth was 39.15 weeks. The median age at scanning for term controls was 40.7 weeks, which was not significantly different from 39.86 weeks for preterm infants.
Inter- and Intraobserver Correlation
With the analysis of agreement, intraobserver correlation coefficient (one-way random effects) was 0.93 and interobserver correlation coefficient (one-way random effects) was 0.87 for manual cerebellar quantification measurements. Kendall rank correlation tau-b was 0.9 (95% CI, 0.77 to 1) and 0.8 (P < .0001 for concordance) for inter- and intraobserver correlations, respectively.
Main Outcome Measure
The median cerebellar volume of preterm at term-equivalent age was 25.4 cm3, and that of term infants was 26.9 cm3. On initial analysis, there was a significant median difference of 2.0 cm3 (95% CI, 1.2 cm3 to 2.7 cm3) (2-sided P < .0001). Figure 3 gives the cerebellar volumes of preterm infants at term-equivalent age and those of term controls. However, this graph shows a wide distribution of volumes in preterm infants, with some preterm infants at term-equivalent age having volumes similar to those of term controls and some infants having very small volumes compared with those of term controls. This distribution led to further exploratory analysis.
Box and whisker plot depicting difference in cerebellar volumes of preterm infants at term-equivalent age and term-born controls.
Hemispheres and Vermis Volumes
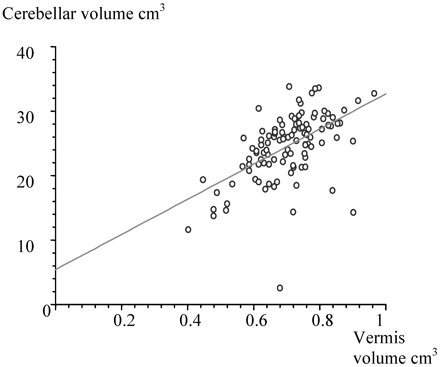
The median cerebellar volume of the left hemisphere was 12.6 cm3 and of the right hemisphere, 12.5 cm3, which was not significantly different as shown in Fig 4. The median vermal volume was 0.7 cm3 and was significantly correlated (r = .5, P < .0001) to total cerebellar volume as shown in Fig 5.
Scatterplot giving the relationship between right and left hemispheric cerebellar volumes.
Scatterplot giving the relationship between cerebellar volume and vermal volume.
Gestational Age
Subgroup analysis of preterm volumes was performed to determine whether any antenatal or postnatal characteristics were related to cerebellar volume in the preterm group. Gestational age at birth did not show any clear correlation with cerebellar volume on linear regression.
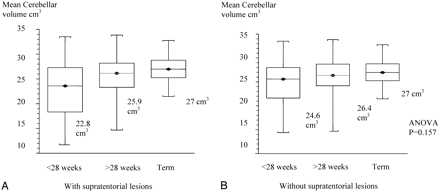
On further exploration, the extremely preterm group of <28-week-gestation infants had a significantly smaller mean cerebellar volume of 22.8 cm3 compared with >28-weeks-gestation infants who had cerebellar volumes of 25.9 cm3 (ANOVA, P = .0025). Term infants had a mean cerebellar volume of 27.0 cm3. Because most of the supratentorial lesions were in the extreme preterm group, when neonates with lesions were excluded, the effect of gestational age failed to reach significance as shown in Fig 6 A,-B.
A, Box and whisker plot of cerebellar volumes of different gestational-age groups with supratentorial lesions.
B, Box and whisker plot of cerebellar volumes of different gestational-age groups without supratentorial lesions.
Cerebellar Volume in the Presence of Supratentorial Lesions
On multiple regression analysis of perinatal variable supratentorial lesions, a P value of .003 was the most significant postnatal factor associated with cerebellar volumes.
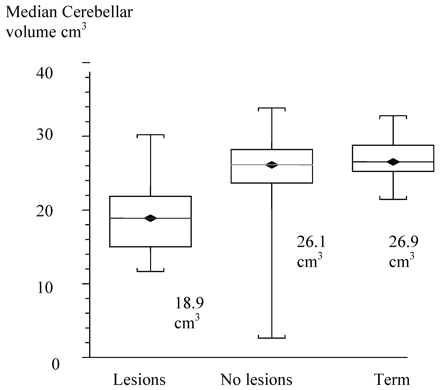
There were 24 infants with supratentorial lesions: 10 cases of periventricular leukomalacia; 8 ventricular dilations, and 6 porencephalic cysts. The median cerebellar volume of infants with supratentorial pathology was 18.9 cm3 (95% CI, 17.4 cm3 to 21.6 cm3). The cerebellar volume of preterm infants who did not have any supratentorial lesions was 26.1 cm3 (95% CI, 24.9 cm3 to 26.8 cm3). This was significantly reduced by 6.4 cm3 (95% CI, −5.0 cm3 to −9.0 cm3) (2-sided P < .0001). The median cerebellar volume of term infants was 26.9 cm3 (95% CI, 25.3 cm3 to 28.8 cm3). Hence, there was no significant difference in cerebellar volumes of preterm infants at term-equivalent age in the absence of supratentorial lesions.
One-way analysis of variance and Tukey-Kramer multiple comparisons of the cerebellar volume of preterm infants with and without supratentorial lesions and of term infants showed significant differences (P < .0001) as shown in Fig 7.
<//B>Box and whisker plot of cerebellar volumes comparing supratentorial lesions versus no supratentorial lesions and term-born controls.
Relationship of Cerebellar and Cerebral Volume
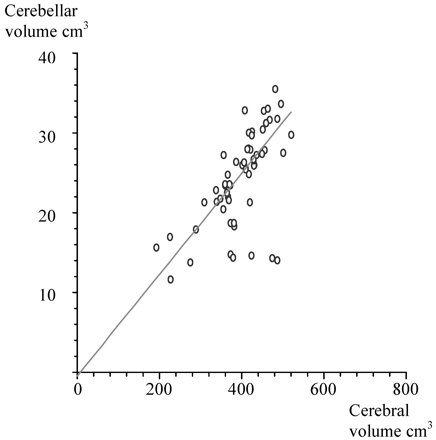
Cerebral volumes were measured in 58 infants (49%). The relationship between cerebellar volumes and cerebral volumes was initially examined by linear regression, which showed significant correlation (Fig 8). However, when the cerebral volumes of infants with supratentorial lesions were examined in relation to cerebellar volumes and vermal volumes, no correlation was found.
Scatter plot giving the relationship between cerebellar volume and cerebral volume.
ANOVA and multiple regressions were performed for the cerebellar volume by using head circumference at scanning, weight at scanning, and cerebral volume entered as independent variables. The linear relationship between cerebellar volume and cerebral volume persisted; however, there was no association of body weight or head circumference at scanning with cerebellar volume. The analysis was also repeated with birth weight and gestational age, which showed some association of birth weight with cerebellar volume.
Indeed, from the multiple regression of all antenatal, perinatal, and postnatal factors, IUGR (P = .03) was the only other factor, in addition to supratentorial lesions, that had a trend toward statistical significance. There were no differences in the volumes between sexes.
Discussion
This study has used a reproducible quantification method for measuring the volume of the cerebellum and has shown that in the absence of supratentorial pathology, premature infants at term-equivalent age have similar cerebellar and vermal volumes, compared with term infants. A reduction in the cerebellar volume was seen in preterm infants with supratentorial lesions. Although no infant had visually obvious cerebellar abnormalities such as hypoplasia and atrophy at term, this study cannot exclude more subtle lesions or small resolved cerebellar hemorrhages that may have been present before scanning or missed by fast spin-echo imaging. Follow-up of these infants would be necessary to determine the relationship between cerebellar volumes and neurodevelopmental impairments commonly seen in ex-preterm infants.
MR imaging is the technique of choice for imaging the cerebellum.22-26 The accuracy of MR imaging–based volumetry has been studied by using a phantom object27 and postmortem samples of cerebellum with a high degree of correlation between MR imaging and specimen results.28,29
Various methods of cerebellar quantification by using manual threshold variations and algorithmic methods have been used in adults.30,31 More novel techniques such as coregistering the scans to standardized coordinates by using a detailed atlas and comparing with postmortem specimens have also been developed.32 However, these are not suitable for the developing immature brain. This study initially used a computerized quantification program for cerebellar volumetry that was developed in our group and validated in adults.33 The program was recently used in term infants with hypoxic ischemic encephalopathy.21 However by with this method, there was inclusion of both cerebral areas and CSF space in most preterm infants at term-equivalent age. Compared with term-born infants, preterm infants at term-equivalent age had less CSF space in the posterior fossa. More over, obliquity of the sections made the accuracy of measurements performed by automated outlining of the cerebellum imprecise. Hence, manual outlining of the cerebellum to quantify the surface area was used and showed good inter- and intraobserver agreements.
We do not have gold standard postmortem values for absolute cerebellar volume at term because only the cerebellum is usually weighed. Cerebellar volumes measured in the fetus by using MR imaging in the first trimester were smaller compared with those of postmortem specimens23; however, it is not known whether the same is true in later gestation. Recently, Chang et al34 measured fetal cerebellar volumes by using 3D ultrasonography in the third trimester, and they found that the volumes are highly correlated to gestational age. In the study by Chang et al, the term infants had a median cerebellar volume of 26.9 cm3, which is similar to findings of the current study.
There is a debate about whether the scaling effect of brain growth needs to be taken into account while measuring cerebellar volumes.35 In mammalian evolution, there is contradictory evidence. One study has shown that the ratio of the neocortex volume to the total brain volume increased, whereas the cerebellum occupied a constant proportion of the total brain volume during evolution.36 However, other studies have shown that through evolution, there was a progressive increase in the cerebellar surface area with increase in cortical surface area, suggesting that the cerebellum and neocortex evolved together,37,38 though the cortical size increased disproportionately.
Hence, in this study, both the absolute difference in cerebellar volume and the difference in volume after accounting for growth of the cerebrum were measured. An absolute difference in cerebellar volumes was found, especially in association with supratentorial lesions. Although there was a linear relationship of cerebral volumes and cerebellar volume, this was altered in infants with supratentorial lesions. Because the cerebral volumes were not measured in all infants, it is possible that there was a type I error in analysis. Moreover, reductions in cerebellar volumes were not associated with body weight at the time of scanning.
Previous studies have shown primary cerebellar hemorrhage or ischemic lesions in preterm infants leading to atrophy.39-41 The cerebellum in term infants is also known to be particularly vulnerable to hypoxic ischemic stresses around the time of birth and vulnerable to malnutrition.42,43 However, in this study of preterm infants, none of the infants had primary lesions at term-equivalent age, and there is no evidence of hypoxic ischemia after birth as suggested by lack of reports of severe hypoxic events in notes, the number of days on ventilator, days on CPAP, or chronic lung disease.
Allin at al,18 studying preterm infants at 14 years of age, found an 11.9 cm3 reduction in cerebellar volume but did not note a clear relation to supratentorial lesions. However, 55% of patients in that study showed other significant generalized MR imaging changes such as ventricular dilation, cysts, and abnormal white matter signal intensity. It is possible that growth during childhood may have obscured early MR imaging of neonatal abnormalities. This possibility could also explain the 12% reduction in cerebellar volumes seen in the study of Allin et al of all infants, compared with the 23% reduction seen in our study in the presence of supratentorial lesions. Further cognitive evaluation of our study infants to determine the outcome of cerebellar abnormalities at term would be valuable.
Alterations in cerebral metabolism and blood flow are seen in brain areas remote to primary injury, termed as “diaschisis” in adult patients with stroke.44,45 Animal experiments have shown that lesions in the forebrain lead to diaschisis and increased apoptosis in remote regions such as the cerebellum.46 Contralateral diaschisis and cerebellar atrophy matching the degree of atrophy in the cerebral cortex have also been reported after stroke, cortical foci, and partial seizures in adults.45,47 A similar phenomenon is seen in infants with hypoxic ischemic encephalopathy, in which reduction in absolute cerebellar volumes of infants with basal ganglia and thalamic lesions has been shown during the first year after delivery.21
The bidirectional cerebrocerebellar pathway may be the anatomical basis of the cerebellar cognitive syndrome.16,48 Neuroimaging in preterm infants has shown brain abnormalities, including reduced myelination, reduction in cortical gray, white matter volume, and reduced complexity of cortical folding, in addition to classic features such as intraventricular hemorrhage and periventricular leukomalacia49,50; however, details about the cerebrocerebellar pathway in preterm infants are not known. Preterm infants have reduction not only in cortical folding but also in regions of deep gray matter.51,52 It may be that alterations in the corticothalamocerebellar connections lead to alterations in cerebellar growth.
The infant’s brain has great plasticity, which is shown by regrowth and the presence of hypertrophy of tracts after perinatal injury in the brain.48,53 The neonatal cerebellum weighs approximately 18–20 g, which is only 5%–6% of the brain weight compared with 10% in adults.54 Although abnormalities in volume, surface area, and microstructure of cortex, white matter, and central gray matter structures49,51,52 have been documented in preterm infants at term-equivalent age in the absence of supratentorial lesions, this study shows preservation of cerebellar growth in these infants. This preservation may be the result of greater plasticity offered by the relatively late development of the cerebellum postnatally, with continuing cell differentiation and migration compared with the cerebral cortex.20 Indeed, during the last trimester and first year of life, it has been shown that the growth velocity of cerebellar volume exceeds that of total brain growth.20,21 On the other hand, it is possible that the cerebellar growth velocity during early childhood may be diminished in preterm infants with initially normal volumes, leading to smaller volumes seen later in life.18
Conclusion
This study shows that preterm infants at term-equivalent age with normal imaging findings have cerebellar and vermal volumes similar to those of term-born controls. However, there is a reduction in cerebellar volume in the presence of supratentorial lesions. This reduction may be due to a primary cerebellar injury that is not detectable by MR imaging at term-equivalent age or due to wallerian degeneration secondary to supratentorial lesions.
References
- Received April 1, 2005.
- Accepted after revision August 1, 2005.
- Copyright © American Society of Neuroradiology