Abstract
BACKGROUND AND PURPOSE: Intra-arterial fibrinolytic therapy is a promising treatment for acute ischemic stroke. Few data are available on its use in elderly patients. The purpose of this study was to compare the baseline characteristics, complications, and outcomes between intra-arterially treated ischemic stroke patients aged ≥80 years and their younger counterparts.
METHODS: Patients aged ≥80 years (n = 33) were compared retrospectively with contemporaneous patients aged <80 years (n = 81) from a registry of consecutive patients treated with intra-arterial thrombolysis over a 9-year period.
RESULTS: The very elderly and younger cohorts were very similar in baseline characteristics, including pretreatment stroke severity (National Institutes of Health Stroke Scale [NIHSS] 17 versus 16), differing only in history of stroke ransient ischemic attack (42% versus 22%, P = .01) and weight (66.8 versus 75.8 kg; P = .02). Significant differences in recanalization (TIMI 2–3) rates could not be detected between the very elderly and younger patients (79% versus 68%, P = .10). Rates of major symptomatic hemorrhage (7% versus 8%) and any intracerebral hemorrhage (39% versus 37%) did not differ. Outcomes at 90 days showed lower rates of excellent functional outcome (mRS ≤1, 26% versus 40%, P = .02) and survival (57% versus 80%, P = .01) among the very elderly.
CONCLUSIONS: Intra-arterial fibrinolysis in the elderly can be accomplished with recanalization rates and hemorrhage rates equal to that in younger patients. Although mortality rates are higher and good functional outcomes are lower than in younger persons, nondisabling outcomes may be achieved in a quarter of patients. These findings suggest that the investigation and use of intra-arterial thrombolytic treatment in very elderly patients should not be avoided but pursued judiciously.
Stroke is a strongly age-related disease, and the number of elderly persons is rapidly increasing.1–3 Per capita, persons aged 85–94 years experience a 2-fold higher incidence of stroke compared with persons aged 65–74 years and a 26-fold higher incidence compared with persons aged 45–54 years.1 The very elderly population is rapidly growing worldwide. In the United States, the population of persons aged >65 years is projected to double to more than 79 million by the middle of this century.4,5 A dramatic increase in the number of elderly stroke patients is foreseen over the coming decades. At current incidence rates, the number of persons aged >85 years experiencing stroke annually will increase from 46,000 in the year 2000 to 390,000 in the year 2050. Advances in acute therapies for strokes in the elderly are urgently needed.
Intra-arterial (IA) fibrinolysis is a promising ischemic stroke treatment. However, its safety and efficacy in elderly persons has not been well explored. In the PROACT randomized clinical trials of (IA) pro-urokinase, very elderly patients (≥85 years) were excluded, and age-specific outcomes for patients >80 years were not reported.6,7 Although some reports have described treatment with conventional intravenous tissue plasminogen activator (tPA) in the very elderly,8–11 no large series has directly examined age-specific results of IA treatment in the elderly.
The purpose of this study was to identify age-specific aspects of response to IA fibrinolytics by retrospectively analyzing a large cohort of patients who have had ischemic stroke who were treated at our institution with local IA fibrinolysis, comparing baseline characteristics, treatment variables, and outcomes between very elderly and younger patients.
Subjects and Methods
Patient Selection
Data were retrospectively collected and analyzed for all patients receiving IA fibrinolytic therapy from July 1992 through December 2001 at our institution. IA thrombolytic therapy was administered as part of a clinical trial12 in 1 patient and on a compassionate care, off-label basis in the remainder. Time from symptom onset was the initial consideration for taking a patient to the neurangiographic suite. Decision to take patients presenting beyond 6 hours from symptom onset was based on evidence of substantial salvageable penumbral tissue on multimodal MR imaging or CT. IA thrombolysis was considered whenever a patient presented with acute cerebral ischemia and the patient had a major cerebral vessel occlusion on digital subtraction angiography. There was no absolute upper or lower age limit for treatment. The decision to treat a patient with IA thrombolysis was made jointly by the attending stroke neurologist and interventional neuroradiologist. Elderly patients were treated starting in 1996. Imaging evidence of an acute or subacute intracerebral hemorrhage was the only absolute exclusion criterion. Evidence of early infarct signs on head CT in more than a third of the middle cerebral artery territory was variably considered to be a contraindication for therapy, depending on attending physician preference. Our local Institutional Review Board approved analysis and reporting of results.
Angiographic/Thrombolytic Procedure
Cerebral angiography was performed via a transfemoral approach. A diagnostic angiogram was obtained to document an arterial occlusion corresponding to the patient’s symptoms before the start of IA treatment. Patients received 1 of 3 treatment regimens: 1) combined IV-IA tPA, with the IV portion administered at a dose of 0.6 mg/kg (10%–15% bolus, remainder over 30 minutes) started within 3 hours of the last known well time, followed by IA tPA up to a maximum dose of 22 mg, 2) only IA urokinase up to a total dose of 1,250,000 IU, or 3) only IA tPA, generally up to a total dose of 22 mg. IV heparin was generally administered at the start of the procedure at doses determined by the interventional neuroradiologist’s preference.
The thrombolytic agent was delivered through a microcatheter up to 6 hours from symptom onset for the anterior circulation and up to 24 hours for the posterior circulation. In general, a small dose of lytic agent was delivered distal to the thrombus, the catheter was repositioned, a small dose of lytic agent was infused directly into the thrombus, the catheter was again repositioned, and the bulk of the dose was infused proximal to the occlusion. Mechanical thrombus disruption was performed by passing the catheter through the thrombus several times when deemed appropriate. In select cases, angioplasty was also performed to treat residual stenosis or to achieve recanalization in unresponsive cases. Diagnostic angiograms were completed at regular intervals to assess recanalization. The thrombolytic infusion was discontinued when full recanalization was achieved, when partial or no recanalization occurred but a maximal safe dose of thrombolytic drug had been administered, or when hemorrhagic transformation was suspected. The angiographic result was graded by the treating physician according to the Thrombolysis in Myocardial Infarction (TIMI) and Mori scales.13,14
Clinical Variables and Outcome Measures
Demographic, clinical, and laboratory data for 57 variables were recorded for each patient. Outcome measures examined included in-hospital death, survival to day 90, National Institutes of Health Stroke Scale (NIHSS) score at 24 hours and at day 7, and the modified Rankin scale (mRS) score at days 7 and 90. A total of 154 data points were missing. One patient from each group was lost to follow-up, in that up to the time of this writing, we were unable to contact these patients and they were not seen in our clinic at any time after discharge.
Imaging Techniques
Patients generally underwent head CT scanning before angiography, immediately after the thrombolytic procedure, at 24 hours after treatment, and if clinical worsening occurred. Frequently, MR imaging scans were obtained in addition to, or instead of, CT scans.
Symptomatic status of hemorrhagic transformation (HT) was classified in the following manner: 1) asymptomatic HT (no clinical worsening on NIHSS score despite HT, 2) minor symptomatic HT (hemorrhagic transformation associated with a 1–3-point increase in the NIHSS score),15 3) major symptomatic HT (hemorrhagic transformation associated with a ≥4-point increase in the NIHSS score or a 1-point deterioration in level of consciousness7), and 4) any symptomatic HT (hemorrhagic transformation associated with any clinical worsening on the NIHSS, either major or minor).
Statistical Analyses
Patients were stratified into 2 age groups: ≥80 and <80 years. Univariate comparisons were conducted with χ2 test or Fisher exact test, 2-tailed, for dichotomous variables, parametric analysis of variance (F) test for normally distributed variables, and the nonparametric Kruskal-Wallis test for non-normally distributed variables. A multivariate logistic regression model of predictors of outcomes (good functional outcome of mRS 0–2 and death) to assess whether age, considered both as a continuous variable and dichotomized at 80, was an independent predictor for outcomes. The relation between age and outcome was further studied by dividing the cohort into 4 age quartiles.
Results
A total of 114 patients received IA fibrinolytic therapy for acute cerebral ischemia during the study period. Of the cohort, 33 (29%) were very elderly (aged ≥80 years) and 81 (71%) were nonelderly. The mean age for the very elderly group was 86 years (range, 80–97 years) and that for the nonelderly was 64 years (range, 25–79 years). Baseline patient characteristics for the 2 groups are presented in Table 1. Of the 57 baseline characteristics measured, only history of prior stroke ransient ischemic attack (TIA) and weight differed significantly between the 2 groups. The very elderly were more likely to have a history of prior stroke/TIA (42% versus 22%, P = .03) and to weigh less (67 versus 76 kg, P = .02). Median pretreatment NIHSS score was 17 for the very elderly and 16 for the nonelderly.
Baseline characteristics of patients with acute ischemic stroke treated with IA thrombolysis
Treatment variables did not differ significantly between the 2 groups, including no differences in time from last known well to treatment start and frequency of combined IV-IA versus IA therapy alone (Table 2).
Treatment variables of patients with acute ischemic stroke treated with IA thrombolysis
Recanalization rates (TIMI 2 or 3) were at least as good in the very elderly (79% versus 68%, P = .25). Across the entire cohort, hemorrhagic transformation occurred in 43 patients (38%). The incidence of any cerebral hemorrhage, asymptomatic hemorrhage, minor symptomatic hemorrhage, and major symptomatic hemorrhage did not differ between the 2 groups (Table 3). There was also no difference between the elderly and nonelderly in hemorrhage location; equal percentages suffered lobar and deep (basal ganglia halamus). The elderly did not have a significantly higher incidence of hemorrhages in territories outside of the zone of infarction.
Hemorrhage rates
At day 7, the median NIHSS score was 19 for the elderly and 12 for the nonelderly (P = .01). Mortality during the initial hospitalization was increased 2-fold among the very elderly compared with younger patients (18% versus 10%, P = .01). The very elderly continued to have an increased risk of dying after hospitalization; only 57% of very elderly patients survived to day 90 compared with 80% of the nonelderly (P = .01).
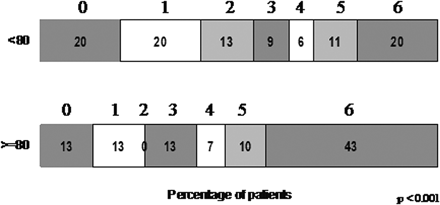
However, a substantial proportion (26%) of the elderly patients achieved an excellent final day 90 outcome (mRS 0–1), though less than younger patients (40%, P = .02; Fig 1). Thirteen percent of very elderly patients had complete recovery with no residual symptoms (mRS = 0). The proportion of patients with a severely disabled (mRS 4–5) final outcome did not differ between the very elderly and younger patients (17% versus 17%).
Day 90 modified Rankin scores in patients aged ≥80 years versus younger patients.
In multivariate analysis, age considered as a continuous variable was an independent predictor of death at day 90 (odds ratio [OR], 1.069; confidence interval [CI], 1.021–1.118; P = .004), along with failure to recanalize (OR, 0.269; CI, 0.099–0.731; P = .01) and pretreatment warfarin use (OR, 0.218; CI, 0.043–1.101; P = .076). The multiple logistic regression model incorporating these variables had a receiver operating characteristic curve (ROC) area of 0.754 and will accurately predict death approximately 75% of the time. In multivariate analysis, age considered as a continuous variable was also an independent predictor of good functional outcome at day 90, along with absence of prior history of stroke/TIA, pretreatment NIHSS score, warfarin use, and highest systolic blood pressure during treatment (Table 4). The multiple logistic regression model incorporating these variables had an ROC area of 0.848 and will accurately predict good functional outcome approximately 85% of the time.
Multivariate predictors of good functional outcome (mRS 0–2) (with age as a continuous variable)
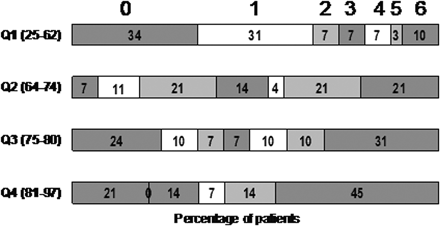
In multivariate analysis, age considered as a dichotomous variable with the division point at 80 years was not a predictor of death or good functional outcome. Graphical analysis of the relation between age quartiles and outcome (Fig 2) suggested a more marked drop in good outcomes at above age 63 than above age 80, and a steady increase in likelihood of fatal outcome across all quartiles.
Day 90 modified Rankin scores by age quartiles.
Discussion
In this large cohort, IA fibrinolysis in patients aged ≥80 years yielded recanalization rates and HT rates equal to that in younger persons, a lower proportion of good functional outcomes, and a higher rate of fatal outcomes.
The rate of recanalization in our series was similar to that achieved in PROACT II and in previous IA cohort series reports.7 Although no prior study specifically examined recanalization in the very elderly, our findings accord with that of Arnold et al16 who did not find a general association between age and recanalization. Our findings suggest that well-described age associated alterations in intrinsic clotting and fibrinolytic systems17–20 do not alter thrombi responsiveness to locally administered fibrinolytic agents in the very old.
Several factors have been postulated to increase the risk of intracerebral hemorrhage after fibrinolytic therapy in elderly patients, including cerebral amyloid angiopathy,17,21 fragile vasculature, and impaired rate of tPA clearance.18,22 Several studies have identified age as an independent risk factor for intracerebral hemorrhage after intravenous fibrinolysis, administered for both myocardial and cerebral ischemia.23–25 However, the risk of HT after IA fibrinolysis was not higher in the very old in our series. Although it did not specifically examine the elderly, the PROACT II trial26 and the large series of Arnold et al16 similarly found that increasing age was not an independent predictor of symptomatic hemorrhage. It has been shown previously that independent predictors for HT after IA lysis include presenting NIHSS score, platelet count, time to recanalization, and serum glucose level, but not age.12 There are several potential explanations for the absence of a relationship between age and HT in ours and prior series, including more careful selection of very elderly patients for intervention and less effect of age-related hemorrhage propensity factors when lytic agent is administered locally rather than systematically. In addition, prior IA studies and our study were adequately powered only to exclude a major, not modest, effect of age on hemorrhage risk.
The lower rate of good functional outcome and higher mortality rate observed in the patients aged ≥80 years after IA lytic therapy accord with many prior studies showing reduced rates of good outcome and higher mortality in elderly than in nonelderly patients treated with conventional, noninterventional therapies and with intravenous tPA.16,27–29 In our series, these outcomes are clearly not due to a poorer recanalization rate or to a higher risk of intra-cranial hemorrhage. A variety of factors probably contribute to reduced functional outcome, despite successful reperfusion in the elderly, including a higher frequency of prior stroke/TIA, higher frequency of prestroke comorbid conditions and poststroke medical complications, impaired collateral circulation, reduced neuronal reserve, and fewer social supports.
Is IA fibrinolytic therapy worth pursuing in patients aged ≥80 years in clinical trials and compassionate care practice, despite the lower rate of good outcomes and increased rate of mortality? This question cannot be answered definitively by an open case series study. Our own impression is that the outcomes observed compare favorably with how these patients would have fared under noninterventional care, but a randomized trial is required to confirm or disconfirm this impression. One important insight offered by our findings is that the frequency of severely disabled outcomes is not increased by pursuing an interventional course. A common concern of patients, families, and physicians is that aggressive intervention in the very elderly will increase the occurrence of severely disabled outcomes that many consider a worse outcome than death.30 Patients and providers often prefer that treatment options will result either in good functional outcome or in death but not in an intermediate state of severe disability. Our findings suggest that IA fibrinolytic therapy in the very elderly does not result in an increased frequency of severely disabled outcomes compared with younger persons and achieves good functional outcome in a quarter of patients. These findings can be used in informing patients and families during compassionate care decision-making and in the design of clinical trials in patients aged 80 years or older.
Limitations of the present study include the retrospective, single center design. The treatment approaches used were somewhat heterogeneous, including different thrombolytic agents and routes over a 9-year period, but treatment strategies did not differ in patients aged ≥80 years and their younger counterparts.
Conclusion
Our results indicate that recanalization is achieved with IA fibrinolytics as frequently in very elderly patients as in younger patients, without higher risks of hemorrhage. Although mortality is higher, a substantial minority of the patients aged ≥80 years achieved good outcomes with IA thrombolysis. Further investigation of IA fibrinolytic therapy in the very elderly is merited to develop improved methods to identify, before therapy starts, which patients are capable of achieving a good functional outcome if recanalization is achieved. Furthermore, our data suggest that patients aged 80 years or older should not be excluded from acute stroke trials using thrombolytic drugs or other strategies.
Acknowledgments
UCLA Intra-Arterial Thrombolysis Investigators: Doojin Kim, MD; Gary A. Ford, FRCP; Chelsea S. Kidwell, MD; Sidney Starkman, MD; Fernando Vinuela, MD; Gary R. Duckwiler, MD; Reza Jahan, MD; Pablo Villablanca, MD; Paul M. Vespa, MD; Megan C. Leary, MD; Margaret Tremwell, MD; Bruce Ovbiagele, MD; Jeffrey L Saver, MD.
Footnotes
This study was supported in part by grants K24-NS02092 (to J.L.S.), K23-NS02088 (to C.S.K.), and P50-NS044378 (to J.L.S., C.S.K., S.S., R.J., D.K.), from National Institutes of Health-National Institute of Neurological Disorders and Stroke.
References
- Received December 16, 2005.
- Accepted after revision March 2, 2006.
- Copyright © American Society of Neuroradiology