Abstract
BACKGROUND AND PURPOSE: To systematically analyze conventional angiographic (CA) features of children with primary central nervous system angiitis (cPACNS), to compare and correlate CA and MR angiography (MRA) lesion characteristics, and to define the sensitivity and specificity of MRA with CA as a reference standard.
METHODS: A retrospective, single-center cohort study of consecutive patients with cPACNS was performed. Patients with CA and MRA studies at diagnosis were included. Imaging studies were blindly reviewed by 2 neuroradiologists using a standard analysis protocol. CA and MRA studies were compared using nonparametric analysis.
RESULTS: Of 45 patients with MRA at diagnosis, there were 25 for whom CA and MRA studies were performed within 1 month of each other. These comprised the study group. The CA distribution of lesions was multifocal (76%) and proximal (86%) (P < .05) with a trend toward unilaterality (P = .06) with anterior circulation involvement (P = .08). The sensitivity and specificity of MRA for CA abnormality was 70% and 98%, respectively. There was no significant difference between MRA and CA for lesion detection or characterization (P = .87), and the modalities showed a fair correlation (κ = 0.4).
CONCLUSION: Angiographic lesions are multifocal and occur proximally and unilaterally within the anterior circulation. There is no significant difference in the ability of MRA to detect and characterize lesions when compared with CA.
Primary central nervous system angiitis of childhood (cPACNS) is a severe and ill-defined disease entity. The diagnosis requires the exclusion of an underlying condition known to be associated with secondary central nervous system (CNS) vasculitis, such as infections, sickle cell disease, Moyamoya disease, systemic vasculitis, migraines with vasospasm, and rare metabolic vasculopathies.
Because brain biopsy is seldom performed in the pediatric age group, the diagnosis of cPACNS depends on clinical assessment and imaging characteristics. Neuroimaging techniques include MR imaging, conventional angiogram (CA), and MR angiogram (MRA). Before the introduction of good quality CT angiography, CT scans were generally not helpful to detect active PACNS without infarction. A combination of MR imaging and CA are commonly used to assess parenchymal and vascular abnormalities. A recent study reported the high sensitivity of MR imaging in a large pediatric cohort of PACNS.1
The principal modalities for vascular imaging used in cPACNS are MRA and CA. CA is not without risk and carries a false-negative rate of up to 44% in the adult PACNS population. Most commonly, arteriosclerosis has been shown to mimic CNS vasculitis.2 However the mimics of childhood PACNS are different and include thromboembolic events, fibromuscular dysplasia, and dissections.
Since its introduction, MRA has been explored as an alternative to conventional angiography in the detection and characterization of lesions.3–5 Several authors have used various MRA techniques to determine the accuracy of MRA to detect intracranial stenosis with variable results.6–9 Although the angiographic pattern of PACNS in adults has been well described,2,10–13 no systematic analysis is available in children.13,14–21
The aims of this study were to 1) systematically analyze CA features of consecutive children with cPACNS, 2) compare CA characteristics and findings on corresponding MRA studies, and 3) define the sensitivity, specificity, predictive values, and correlation of MRA findings in relation to CA.
Materials and Methods
Study Cohort
Institutional Review Board approval was obtained for this single-center retrospective review of consecutive children (2 months–18 years old) with cPACNS diagnosed between January 1, 1990, and December 31, 2001. A search in the Hospital for Sick Children (HSC) Rheumatology data base and the Canadian Pediatric Ischemic Stroke Registry (Toronto site) using keywords “CNS vasculitis” or “CNS angiitis” was cross-referenced with an International Classification of Diseases (ICD-9) code search for discharge diagnoses “vasculitis,” “CNS vasculitis,” and “CNS angiitis” in the HSC health records data base.
Inclusion criteria were 1) clinical diagnosis of primary CNS angiitis of childhood, 2) angiographic confirmation of suspected diagnosis cPACNS, and 3) corresponding CA and MRA studies less than 1 month apart at time of diagnosis. The study excluded 1) neonates, 2) patients with underlying causes of CNS vasculopathy (eg, chickenpox less than 12 months before disease onset, sickle cell disease, Moya/moya disease, migraines, systemic lupus erythematosus) and 3) those with incomplete imaging.
Forty-five consecutive patients with cPACNS were screened. Thirty-two patients had MRA and CA studies performed during the initial admission, but only 25 patients had the 2 studies within 1 month of each other. These patients constituted the study group. There were 16 boys and 9 girls. The median age at presentation MRA and CA was 7.6 years (0.8–16.5 years). The time interval between CA and MRA was a median of 5 days (range 1–30).
MRA Technique
MR imaging was performed on a 1.5T superconducting magnet (Signa EchoSpeed software, ver. 8.2.3; GE Medical Systems, Milwaukee, Wis). Apart from standard MR imaging sequences 3D time-of-flight (TOF) MRA was performed (34/4.8, 30 TR/TE/flip angle; matrix of 256 × 256; and FOV of 14–15 cm). Magnetization transfer, ZIP2, and ZIP 512 (zero-fill interpolation processing) were used. 3D TOF was divided into 3 slabs with 30% overlap. Each slab comprised 64 partitions with an overall section width of 1 mm. Coverage from the foramen magnum to the pericallosal artery was obtained. Maximum intensity projection (MIP) images rotated 15°–20° in each direction were reviewed on hard copy or PACS where available. Studies before 1997 differed from those thereafter in terms of the window presentation and were imaged as black vessels on a white background. Source images were not available for hard-copy MRA studies and therefore were also not reviewed for PACS studies to prevent bias.
CA Technique
Biplane angiography was performed under general anesthesia by a single operator in all cases. A LCN biplane (GE Medical Systems) was used. Standardized views were performed in all cases. For the anterior circulation, these include anteroposterior, lateral, anteroposterior oblique (15°, 30°, and 45°); for posterior circulation, Townes, lateral, and bilateral 45° anteroposterior obliques. Additional views were taken in the context of abnormality. The images were reviewed on hard copy or PACS where available.
Imaging Review
MRA and CA studies were blinded. Reading was performed in random order. Standardized reviews of MRA and CA were performed by 2 study neuroradiologists. All assessments were conducted by consensus between the 2 reviewers. A standard analysis protocol was developed. MIP MRA images were evaluated for stenosis. The vascular segment was allocated according to standard anatomic texts.22,23
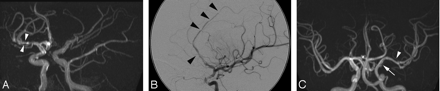
Stenosis was measured using the calibration markers on each image. The projection showing the tightest stenosis was chosen for each lesion with measurements made from the outer margins of the vessel wall. The intracranial vessels were assessed according to predesignated divisions and whether proximal, distal, or whole segments were involved. Where an entire vessel was abnormal, assessment of stenosis was made by comparing the vessel size with that of the nearest normal vessel. The degree of stenosis (occlusion 100%) and morphology of each stenosis were recorded. Standardized morphologic definitions24 were: concentric, eccentric, graduated, smooth, irregular, dilation, and aneurysm. Beading (Fig 1A) was defined as alternating short regularly spaced segments of stenosis with short normal or dilated intervening segments. Involvement of a single vessel segment with multiple stenoses separated by a gap greater than seen with beading was recorded as “multiple” (Fig 1B). A “benign-appearing” configuration (Fig 1C) was defined as 3 or more of the following: single, concentric, smooth, and graduated. “Aggressive appearance” (Fig 1A,-B) was defined as 2 or more of the following: irregular, multiple, and eccentric. Distal involvement was assessed by involvement beyond the M1 or A1 segment. The presence or absence of collateral supply was documented.
Representative MRA and angiographic images. Aggressive appearing lesions were those that demonstrated multiple short segment irregularity with alternating narrowing and dilation (beading) (white arrow) (A) or multiple longer segmental narrowing with normal intervening vessel (black arrowheads) (B). Aneurysms (not shown) were included in this definition. Benign appearing lesions (C) had smooth (white arrows), often solitary, tapered (white arrowhead) narrowing that could be concentric or eccentric. Incidental hypoplasia of the ipsilateral A1 is noted.
Where an absent or diffusely smooth A1 segment of the anterior cerebral artery or posterior communicating segment was seen this was documented as hypoplastic (Fig 1C). The same approach was adopted for the P1 segment in a fetal posterior cerebral artery arrangement.
Analysis
Data were transferred into a dedicated data base. Proportions for MRA and CA detected morphologic abnormality (location, multiplicity, morphology, and vascular distribution of stenosis) were calculated using Fisher exact test. Significance was assigned at P < .05. Sensitivity, specificity, and positive and negative predictive values were calculated on a per-lesion and per-patient basis for MRA. Agreement between MRA and CA was calculated using κ, a measure of observer agreement. All analyses were performed with SPSS 10 (SPSS, Chicago, Ill).
Results
CA Appearances
Twenty-one of 25 (84%) patients had an abnormal CA; thus, 16% of patients had a normal CA in the context of cPACNS. Sixty-four lesions were identified (median, 2; range, 1–6). Lesions were significantly more likely to be proximally distributed (P < .05); there was a proximal lesion in 86% of cases. There was a trend to involve the anterior circulation (P = .08), to be unilateral (90%) (P = .06), and to be multifocal (P = .09) (Table 1). None of the patients had disease of M3/4, P3/4, or pericallosal segments without proximal disease. The distribution and morphology of CA lesions is listed in Tables 2 and 3. Most lesions occurred within the middle cerebral artery (MCA) (41%) with proximal predominance (62%). Anterior cerebral artery (ACA), internal carotid artery (ICA), and posterior circulation involvement occurred in descending order of frequency (27%, 17.5%, and 14%, respectively). Lesions were more likely to have features suggestive of a benign-appearing pattern of stenosis (Tables 1 and 3) ie, smooth (83%), concentric (93%), and single (89%); 24% and 76% of patients overall showed aggressive and benign-appearing features.
Demographic distribution of abnormality in patients with abnormal MRA and CA
Distribution of lesions on MRA and CA
Morphology of CA and MRA stenoses
CA versus MRA
Twenty-four patients (96%) had an abnormal MRA. Of these, 20 had an abnormal CA. One patient had normal CA 3 days after the abnormal MRA and developed a CA abnormality 70 days later. Three patients with abnormal MRA had normal CA initially and were subsequently followed by MRA without further CA imaging. The patients had a clinical course consistent with vasculitis, and the MRA appearances resolved over a period between 2 months and 1 year, corresponding to clinical improvement.
Conventional angiography detected 54 stenoses, 8 occlusions, and 2 aneurysms, whereas MRA detected 56 stenoses, 4 occlusions, and 2 aneurysms. The distribution and morphology of MRA and CA abnormality is presented and compared in Tables 1–3.
Lesion Identification
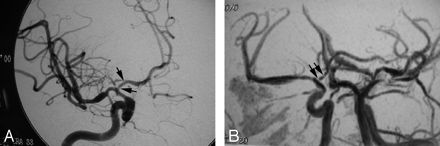
MRA correctly identified 45 of 64 angiographically detected lesions, yielding a sensitivity of 70% and accounting for only a fair agreement between the 2 modalities (κ = 0.4) (Fig 2). There were 18 false-positive MRA observations. MRA was normal in 701 vessel segments, yielding a specificity of 98%. The positive and negative predictive values (PPV, NPV) were 71% and 97%, respectively. There was no significant difference between MR and CA for lesion detection (P = .87).
Good agreement of CA (A) and (B) MRA for right ICA. Both modalities demonstrate tapered narrowing of the terminal carotid and proximal M1 and A1 (carotid terminus) with focal midM1 dilation and distal narrowing. Both modalities identify beading of the proximal A1 (black arrows).
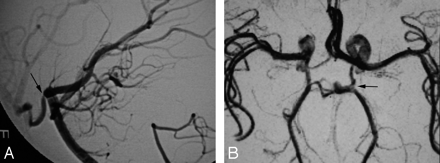
Considering patients rather than lesions, false-negative segments occurred on MRA in 7 patients with 19 lesions. Only 1 patient with an abnormal MR imaging had a normal MRA and an abnormal CA. The angiographic abnormality was in the left posterior communicating artery (PcomA) (Fig 3). The remaining patients had other MRA and CA abnormalities that would have suggested the diagnosis without necessity for MR imaging review. The overall sensitivity for diagnosis per patient was 95% with a PPV of 83%. Specificity and NPV could not be calculated. The distribution of MRA false-negative segments is presented in Table 4, showing higher frequency in the distal (M2) segments and ACA (21% each) followed by the cavernous portion of the ICA (16%).
Abnormal CA (A) in the context of normal MRA (B) in a patient with a lone focal stenosis of the left PcomA. No other CA abnormality was present. MR imaging was abnormal maintaining suspicion for vasculitis despite a negative MRA.
Vascular segmental location of MRA false-negatives
Assessment of Stenosis
We next compared the degree of stenosis as detected by CA and MRA. As can be seen in Table 5, there was an overall agreement of 43 of 62 lesions between CA and MRA; the correlation improved with increasing severity of the stenosis. Approximately half of the MRA false-negatives occurred when the stenosis on CA was <50%. Only 20% of the CA lesions of <50% were correctly classified on MRA. For lesions between 50% and 75%, the agreement was 48%, with 6 lesions (24%) considered to be in the next higher grade and 1 a grade lower (4%) on MRA. Stenosis of 75%–99% was correctly graded in 76.5% of cases with MRA; 1 lesion was misinterpreted as occlusion.
Stenosis quantification
Discussion
This series describes the CA findings in the largest single-center cohort of patients with PACNS and assesses the agreement of MRA with CA for lesion characterization and localization. We also review reported cases that number fewer than 25 children, reported in several case studies, letters, and abstracts.14–21
Our results indicate that childhood PACNS is characterized by proximal, multifocal, and unilateral involvement within the anterior cerebral circulation. MRA performed comparably with CA for lesion characterization and localization. CA detected more lesions than MRA, resulting in a fair correlation.
Conventional Angiography
Angiography is considered the radiologic “gold standard” in diagnosing patients with vasculitis. It is invasive, usually requiring a general anesthetic in the pediatric population, and carries a small but significant risk of stroke and femoral artery thrombosis. This risk may be higher in children having angiography for investigation of stroke. Ganesan et al,3 reporting on angiography in 46 pediatric patients (median age, 6 years and 7 months) with stroke, described a single case (2.2%) of angiography-related occlusion of the MCA. None of the patients in our cohort developed a stroke following angiography. The rate of angiogram-negative patients with PACNS in our cohort was 16%, which is lower than the reported rates of 20%–40% in adults. A negative CA is presumed to be caused by its inability to detect abnormality in small vessels.2,10,13,25,26
The angiographic appearance of angiitis varies and is nonspecific due to a wide range of vasculitis mimics, including vasospasm, vascular ectasia, radiation, drug exposure, and infection.24,27–30 A recent study in adults31 confirmed the poor specificity of angiography against biopsy; none of 14 cases with angiographic features was typical for vasculitis demonstrating biopsy proof of the condition. However, this has rarely been reported to occur in children, and in our study, alternate diagnoses were excluded with rigorous clinical and laboratory tests and where the ensuing clinical history and laboratory findings were typical for vasculitis.
Distribution
The most common finding reported in adult primary angiitis is short segmental stenosis affecting multiple vessels usually bilaterally and asymmetrically.12,32–35 We report a predominance of unilateral lesions, which agrees with Chabrier16 (8 of 9, 89%) but not in the smaller study by Gallagher14 (2 of 5, 40%). The statistically significant involvement of the anterior vasculature and trend for proximal involvement explains our previous report of more than half the patients presenting with lesions in the basal ganglia within the distribution of the lateral lenticulostriate arteries.1 The MCA and the ACA (frequently limited to the A1 segment) were the most affected vessels concurring with others.33 Chabrier et al16 hypothesized that the perforator vessels may be secondarily occluded by the inflammatory process involving the proximal MCA, which are most susceptible to a reduction in flow from proximal stenosis because of their perpendicular orientation. MRA detected a similar number of distal lesions to angiography because of the paucity of M4, P4, or pericallosal lesions. Distal disease usually coexisted with proximal disease (except in 1 patient with isolated abnormality of the M2 segment of the MCA [4.5%]). Our incidence of isolated or combined posterior and anterior circulation involvement lies in the range of previously reported values (20%–30%).10,14,15
Morphology of Lesions
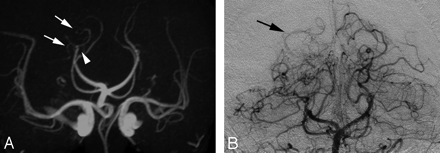
Stenosis was the most common presentation of cPACNS, occurring in 84%. A benign-appearing lesional configuration (Fig 1C) occurred significantly more frequently than an aggressive-appearing or “classic” configuration (Fig 1A,-B). This finding is supported by Calabese et al10 but is contrary to that by Alhalabi et al,12 where equal numbers were described. The value is higher than that obtained by MRA, which is thought to be due to a nonsignificant trend toward underestimating aggressive appearance. Graduated narrowing or tapering of the supraclinoid ICA, described as “frequent” by others,11,12,16,32 is seen in more than half of the patients in our cohort with ICA abnormality. Multifocal lesions (5 or more) were present in nearly 20% of patients. Multiple isolated stenoses occurred as commonly as beading; although better appreciated on CA, the difference was not significant. Similarly, collateral formation was better appreciated on CA (Fig 4).
A, Occlusion of the P3 segment of the right PCA on TOF MRA (white arrowhead). Inferior temporal branches (white arrows) are slightly more prominent than on the contralateral side but B, extent of collaterals and reconstitution of the distal PCA (black arrow) best seen on CA.
Two aneurysms are identified in this study. They are likely to be vasculitic in origin because of their nonsaccular appearance and atypical site. One has features suggestive of a dissecting aneurysm of the P2 portion of the left posterior cerebral artery (PCA), but there was no antecedent history of trauma. Aneurysm or vessel rupture is presumed to be the cause of hemorrhage, an uncommon manifestation of adult and childhood PACNS.12,29,33–38
MRA
A number of studies have evaluated the use of MRA in the detection of intracranial stenosis. 2D and 3D TOF, contrast-enhanced MRA, and phase-contrast techniques have been compared. Tilted optimized nonsaturation excitation and magnetization transfer suppression have been added to TOF sequences to enhance background suppression and maximize signal-to-noise ratios. Maximum intensity projection display has been compared with volume-rendered display.6–9,39–41 A combination of MR angiography and CT angiography has been compared against CA.42 Continued interest in noninvasive imaging against the reference standard of CA implies that CA has yet to be superseded. MRA has the advantage of being a noninvasive, simple, and relatively quick technique with the ability to review the MIP/MPR images in multiple projections, thereby potentially improving lesion detection. However, MRA is limited by resolution and dephasing secondary to turbulence or in-plane flow.7,8,43 Assessment of morphology of a vessel and its branches distal to a high grade stenosis may not be possible. Finally, TOF MRA does not provide information on flow dynamics, such as direction of collateral supply or transit time, and cortical branches are not reliably demonstrated.
It has been suggested that MRA is suitable for detection of proximal vascular lesions associated with basal ganglia infarcts in children with stroke.4,5 Although MRA may not detect lesions of the medium-sized vessels in a third of patients, the overall diagnosis was unaffected because of concomitant proximal disease.4 This group supported the use of MRA in childhood stroke, but they suggested that it may not be suitable for detecting angiitis. However, our data suggested that MRA was able to detect vasculitis in most patients with a positive CA. However, MRA detected fewer lesions than CA.
One study reported PPV and NPV of 100% and 93%, respectively, for MRA detection of hemispheric abnormality in 18 children with idiopathic infarction.5 The authors did not characterize the morphology or degree of stenosis but did attempt to distinguish stenosis from occlusion. Of the 15 abnormal CAs, 13 patients (87%) had proximal steno-occlusive disease (limited to the terminal ICA or proximal MCA), whereas embolus and unilateral multiple vessel accounted for the remaining 2 patients (13%). On a lesion-by-lesion basis, we describe a higher number of false-positives and -negatives, resulting in a lower PPV but a higher NPV. We have demonstrated a high specificity and moderate sensitivity for lesion detection in cPACNS. We report no significant difference between MRA and CA for individual lesion detection and morphologic assessment of lesions. Despite false-positive and -negative MRA segments, overall MRA was able to correctly characterize the distribution (proximal/distal), multiplicity, and laterality (unilateral/bilateral) of lesions compared with CA. In 1 patient, MRA falsely classified the patient as having a single lesion, whereas CA detected 5 lesions. This distinction was important clinically; we have found that a multiplicity of lesions predicts a more aggressive course.44 One patient with an isolated PcomA abnormality was missed on MRA, but the MR imaging was abnormal, alerting the authors to underlying abnormality and leading to a request for a CA. Throughout this study, CA was considered the reference standard, and therefore the 4 patients with abnormal findings in the context of negative CA were treated as false-positive MRA results. Nevertheless, 1 patient developed a CA abnormality 70 days later, and the other 3 had a clinical course consistent with vasculitis with resolution of MRA appearances between 2 months and 1 year on treatment. We can only speculate about the significance of these findings and about whether MRA was in fact more sensitive in the detection of lesions, especially in the patients described.
Stenosis estimation remains challenging on MRA and has implications for assessment of hemodynamic compromise and response to treatment. Limitations of voxel size prohibit accurate MRA estimation of lesions stenosed less than 50%.7,43 Reported sensitivity of MRA for the detection of occlusion in adult series has been reported to be as high as 100%.45 We did not have such a correlation and encountered difficulty distinguishing high-grade stenosis from occlusion in 4 patients. MRA may show an apparent short occlusion yet reveal normal-appearing distal branches, confirming that the lesion is not occlusive. Otherwise, retrograde flow through collaterals may account for this appearance, and flow voids may be absent even in the context of >70% angiographic stenosis.43 Three occlusions were not detected on MRA (1 M4 segment and 2 distal M2 segments). This experience is similar to that of other authors in pediatric series.5
Correct MRA quantification of degree of stenosis greater than 50% in adults varies between 78% and 100%.8,39,46 We had similar agreement for stenosis greater than 75% but poorer agreement in the 50%–75% range, where we overestimated stenosis in 24% of lesions. The tendency to overestimate stenosis on MRA MIP images is well known47 and may be reduced by assessment of the source images.7,8 Allowing for 1 level of stenosis higher in the 50%–99% range, we correctly identify 78% of lesions. Some of our older studies were imaged as black vessels on a white background, whereas most patients were imaged as typically presented today. In the limited subset in which the older display window was used, we did not appreciate any difference in the ability to assess the severity or morphology of stenosis.
MRA does not assess the pericallosal (or A3 portion of the ACA) artery because exclusion from the TOF slab or in-plane flow and cortical MCA branches (M4) are not routinely delineated. Cavernous carotid remains a challenge to stenosis identification for these reasons. Despite rigid definitions, we occasionally had difficulty with long smooth A1 stenoses and a PcomA stenosis, mistaking them for hypoplastic branches. Overall, most false-negative MRA lesions (53%) occurred in these 4 locations.
In our experience, MRA detects abnormality within the proximal intracranial vessels to the level of the proximal M2 or sylvian branches of the MCA and the proximal A2 and P2 branches of the PCA. Nevertheless, most our false-negative MRA (63%) results occurred in these regions. We surmise that this number could have been reduced by review of source data that were not available to us; this remains a limitation of the study.
Conclusion
We have demonstrated proximal, multifocal, and unilateral involvement, predominantly of the anterior intracranial vessels in PACNS.
CA detected more lesions than MRA, but this was not clinically significant. There was no significant difference between MRA and CA for the determination of lesion location and morphology confirming the benefit of MRA in diagnosis. Estimation of degree of stenosis remains challenging but may be improved by reviewing source data together with MPR and MIP images. These findings, combined with our previous experience, lead us to conclude that in the context of an abnormal MR imaging and normal MRA, a CA assessment is indicated. The role of MRA in the follow-up of lesions is unknown and will require prospective MRA and CA studies to be evaluated.
Acknowledgments
We thank Faisel Ahmed for his assistance in data collection and data base administration.
References
- Received February 6, 2006.
- Accepted after revision March 16, 2006.
- Copyright © American Society of Neuroradiology