Abstract
BACKGROUND AND PURPOSE: RTT, caused by mutations in the methyl CPG binding protein 2 (MeCP2) gene, is a disorder of neuronal maturation and connections. Our aim was to prospectively examine FA by DTI and correlate this with certain clinical features in patients with RTT.
MATERIALS AND METHODS: Thirty-two patients with RTT underwent neurologic assessments and DTI. Thirty-seven age-matched healthy female control subjects were studied for comparison. With use of a 1.5T MR imaging unit, DTI data were acquired, and FA was evaluated to investigate multiple regional tract–specific abnormalities in patients with RTT.
RESULTS: In RTT, significant reductions in FA were noted in the genu and splenium of the corpus callosum and external capsule, with regions of significant reductions in the cingulate, internal capsule, posterior thalamic radiation, and frontal white matter. In contrast, FA of visual pathways was similar to control subjects. FA in the superior longitudinal fasciculus, which is associated with speech, was equal to control subjects in patients with preserved speech (phrases and sentences) (P = .542), whereas FA was reduced in those patients who were nonverbal or speaking only single words (P < .001). No correlations between FA values for tracts and clinical features such as seizures, gross or fine motor skills, and head circumference were identified.
CONCLUSIONS: DTI, a noninvasive technique to assess white matter tract pathologic features, may add specificity to the assessment of RTT clinical severity that is presently based on the classification of MeCP2 gene mutation and X-inactivation.
Abbreviations
- AIR
- automated image registration
- ALIC
- anterior limb of internal capsule
- CC
- corpus callosum
- CG1, CG3, CG4
- cingulate gyrus
- CG2
- cingulum
- CP
- cerebral peduncle
- CPG
- cytosine guanosine dinucleotide
- DTI
- diffusion tensor imaging
- EC
- external capsule
- FA
- fractional anisotropy
- FW
- frontal white matter
- FX/ST
- fornix/stria terminalis
- GC
- genu of corpus callosum
- ICBM
- International Consortium of Brain Mapping
- IFO
- inferior fronto-occipital fasciculus
- LI
- laterality index
- MCP
- middle cerebellar peduncle
- PCR
- posterior corona radiata
- PLIC
- posterior limb of internal capsule
- PTR
- posterior thalamic radiation
- ROI
- region of interest
- RTT
- Rett syndrome
- SCC
- splenium of corpus callosum
- SCP
- superior cerebellar peduncle
- SLF
- superior longitudinal fasciculus
- SS
- sagittal stratum.
RTT, a neurodevelopmental disorder that predominantly affects female patients, is caused by mutations in the methyl CPG binding protein 2 (MeCP2) gene located at Xq28.1,2 RTT occurs in 1 in 22,000 girls, and patients with RTT seem to develop normally up to 6 months of life.3,4 Nonetheless, deceleration in the velocity of head growth occurs as early as 4 months of life up to 2 years, leading to microcephaly in most patients.5 Patients lose the ability to use words and experience poor hand use with cognitive and motor deficits.
Pathologic studies show that brain weight in RTT is reduced compared with control subjects.6 This decrease in the weight of the brain is not generalized. It is more significant in the cerebral hemispheres and less prominent in the cerebellum. Brain volume measurements in vivo show regional alterations, with relative preservation in the posterior occipital and posterior temporal regions.7 The regional reduction in volumes is not attributed to atrophy8 but to poor neuronal maturation9,10 and connections.11 Increased cell-packing attenuation because of poor dendritic arborization has been attributed to poor neuronal interconnectivity resulting in microcephaly.6,11,12
Structure-specific pathologic features of white matter can be assessed noninvasively by DTI.13,14 Different quantitative measures can be derived from a DTI study. Our prospective study used the directionality of water diffusion, quantified by FA,15 to investigate the tract-specific abnormalities and their correlations with some clinical features in patients with RTT.
Materials and Methods
Subjects
Thirty-two girls with RTT syndrome were included in the study based on MeCP2 gene mutation analysis and clinical parameters. They underwent neuroimaging and neurologic evaluation as part of the Natural History study in RTT being conducted at the Kennedy Krieger Institute. In-depth clinical assessments included neurologic status, head circumference, history of seizures, respiratory irregularities, gait, and speech. Imaging and clinical tests were performed during the same admission. Thirty-seven age-matched healthy female control subjects were included for comparison. There was no significant age difference between patients with RTT vs control subjects (5.5 ± 0.7 vs 6.1 ± 0.7 years, P = .58). All patients were sedated for MR imaging scan, and control subjects were not.
The Institutional Review Board at Johns Hopkins University approved the study, and informed consent was obtained from legal guardians.
Data Acquisition
A 1.5T MR unit (Philips Medical Systems, Best, the Netherlands) was used for data acquisition. Conventional MR imaging consisted of sagittal T1-weighted, axial T2 fast spin-echo, and axial fluid-attenuated inversion recovery images. Conventional brain MR images were obtained in the patients and control subjects. We acquired DTI data using a single-shot echo-planar imaging sequence with the sensitivity encoding, parallel-imaging scheme (reduction factor, 2.5).16 The FOV was 240 × 240 mm; 96 × 96 imaging matrix was zero-filled to 256 × 256 pixels. Transverse sections of 2.5 mm in thickness were acquired parallel to the anterior/posterior commissure line. DTI was encoded along 30 independent orientations,17 and the b-value was 700 s/mm2. Three DTI datasets were acquired for each participant, and the acquisition time per dataset was approximately 6 minutes.
Data Processing
For DTI processing, software (DTIStudio) built in-house was used (H. Jiang, S. Mori; Johns Hopkins University and Kennedy Krieger Institute, Baltimore, Maryland; http://lbam.med.jhmi.edu).18 We first realigned the images using 12-mode affine transformation of the AIR program for coregistration and eddy-current distortion correction.19 We then calculated the 6 elements of the diffusion tensor for each pixel by using multivariate linear fitting.13 After the diagonalization, 3 eigenvalues and eigenvectors were obtained. For the anisotropy map, FA was used.15
Strategy for Delineation of Region of Interest
The image analysis was based on manually delineated ROIs. To enhance reproducibility of the structure identification, we adopted a method aided by a brain normalization scheme (a “hybrid approach” method) in our previous publication.20 First, all brains were normalized to ICBM-DTI-81 atlas (http://www.loni.ucla.edu/Atlases/Atlas_Detail.jsp?atlas_id = 15) by use of 12-mode affine transformation. To drive the registration, we used non-diffusion-weighted images. This adjusted the overall brain sizes and orientations across the subjects so that 2D observation sections with consistent orientations and locations could be extracted for the ROI drawing. We performed this initial brain registration using Landmarker (www.mristudio.org).20 After the registration, predefined 2D observation places (2 axial section at z = 82 and 65 and 2 coronal sections at y = 89 and 125 in the atlas space) were extracted. In this study, we placed multiple ROIs for large-fiber bundles. These multiple ROIs on the same white matter structures do not always lead to the same observations. The FA values of the same white matter tracts vary along their path.21 Individual axons may merge and exit a tract at many points. It is, therefore, anatomically possible that abnormal FAs are found only at a portion of white matter tracts.21
Fig 1 and the accompanying Table show all 45 ROIs placed at the specific anatomic locations. The shape of these ROIs followed the white matter parcellation map defined in the ICBM-DTI-81 atlas.
Total of 42 ROIs (21 in each hemisphere) and 3 in the corpus callosum are shown.
Comparisons of FA between patients with RTT and control subjects
Statistical Method
The t test and Mann-Whitney test were used for comparison of diffusion parameters between the patients and age-matched female control subjects. A P value of .05 or less was considered statistically significant. To investigate the asymmetry between FA of the left and right hemispheres, we used 2 methods: 1) comparison of left and right FA values, and 2) LI for FA in all examined fiber tracts by using the formula LI = (left − right)/[(left + right)]. Significance of the hemispheric differences was assessed with the paired t test for left-to-right comparison and the unpaired t test for LI comparison. Bonferroni correction was applied. We performed all statistical analyses using STATA version 8.0 (StataCorp, College Station, Texas).
Results
A total of 32 patients with RTT and 37 control subjects were age matched when compared as 1 cohort (P = .58) or in 3 subgroups: 1) younger than 5 years (2.8 [SD, 0.86] vs 2.7 [SD, 1.45]; P = .9), 2) ages 5 to 10 years (7 [SD, 1.09] vs 7.6 [SD, 1.34]; P = .414), 3) older than 10 years (13.5 [SD, 2.72] vs 12.8 [SD, 1.2]; P = .472). Conventional MR imaging of patients and control subjects was obtained, and results were found to be unremarkable in control subjects. However, patients were noted to have microcephaly without other abnormalities. The Table shows the comparisons of FA between patients and control subjects. FA was not different in regions comprising the limbic system, namely the fornix and posterior cingulate gyrus. However, in the patients, FA for the anterior cingulum bundles (CG1) was significantly reduced. Of the cerebellar tracts, only the right middle cerebellar tract showed a trend of reduced FA. Significant reductions in FA were noted in the genu and splenium of the corpus callosum, and external capsule, with regional reductions in the anterior and posterior limbs of the internal capsule, posterior thalamic radiation, and frontal white matter in RTT. In addition, there was no difference in FA between patients with RTT and control subjects in 3 of the 4 ROIs in the posterior thalamic radiation with significantly higher FA in the posterior corona radiata of the patients (Table).
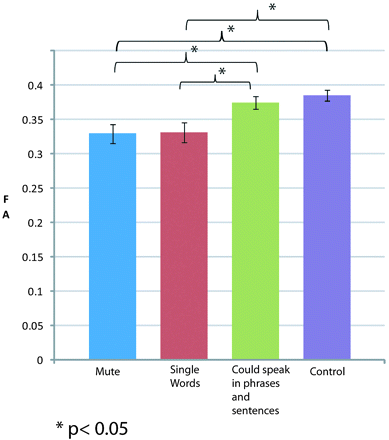
Patients were categorized by their capacity to speak: 1) Mute (n = 13), 2) Single words (n = 13), and 3) Could speak in phrases or sentences (n = 6). As shown in Fig 2, mean FA of the superior longitudinal fasciculus in control subjects and group 3 patients was not different (0.38 ± 0.04 vs 0.375 ± 0.02; t = 0.615; P = .542). In a similar setting, there was no difference in the FA of the patients with RTT in group 1 and group 2 (0.32 ± 0.05 vs 0.33 ± 0.05; t = −0.089; P = .929). But groups 1 and 2 with minimal speech were significantly different from group 3 and the control subjects (group 1 vs group 3: t = −2.12; P = .046; group 1 vs control: t = 3.77; P < .001; group 2 vs group 3: t = −2.03; P = .055; group 2 vs control: t = 3.58; P < .001). This trend was not seen in any other region.
Comparison of FA among patients categorized by speech capacity in the superior longitudinal fasciculus and control subjects.
Our study did not find any correlation between FA values in the examined tracts and seizure status, head circumference, or walking ability. Hemispheric asymmetry analysis evaluating left/right differences showed that, in contrast to control subjects, asymmetry was not present in the inferior fronto-occipital fasciculus (P > .99), external capsule (P > .99), superior longitudinal fasciculus (P > .99), and posterior thalamic radiation (P > .99) in patients with RTT. Additional LI analysis only showed a significant trend in the inferior fronto-occipital fasciculus tracts (P = .082) compared with control subjects. We noted hemispheric asymmetry in CG3 (posterior cingulate gyrus) of the patients (P = .044) compared with control subjects, who showed no asymmetry (P = .505).
Discussion
In the studied group of patients with RTT, the FA was reduced in the corpus callosum, internal capsule, and frontal white matter compared with control subjects. In contrast, mean FA in most of the regions comprising the limbic system and cerebellar tracts was similar in both groups. The posterior corona radiata was the only region in the patients with RTT where FA was greater than that of control subjects and this finding is in keeping with the observation in volumetric studies by Carter et al.7 Without histologic results, it is difficult to conclude about the exact mechanism of the FA decrease observed in this patient population. Past studies have suggested axonal involvement to explain FA reduction,20,22 but loss of myelin has not been shown to be a contributing factor.23 In patients with RTT, axonal vulnerability must be an important contributor to the reduced FA, as shown by reduced levels of N-acetylaspartate in MR spectroscopic imaging.24
We noted 3 major correlations with clinical findings in RTT, the most significant of which was a correlation of FA in the superior longitudinal fasciculus with the ability to speak. The superior longitudinal fasciculus is one of the main association bundles that connect the external surface of temporoparieto-occipital regions with the convexity of the frontal lobe and, in part, is associated with phonologic speech.25,26 The left-more-than-right asymmetry in the superior longitudinal fasciculus, as seen in the control subjects, was not found in the patients.
Second, the presence of intact visual capabilities, which are used by some girls with RTT to communicate, may be in accordance with normal to increased FA in the posterior corona radiata. This observation was in contrast to the FA that was significantly reduced by greater margins in all regions of the corpus callosum affecting interhemispheric connectivity, probably because of immature neurons and their poor dendritic arborization.27 However, other white matter structures that are known to be associated with visual functions such as the sagittal stratum did not show increased FA. The increased FA of the posterior thalamic radiation, therefore, needs careful interpretation with increased numbers of patients. Study data also argue that, with few exceptions, white matter tracts in RTT are diffusely affected compared with control subjects. Except for the right middle cerebellar tract, other cerebellar tracts were generally spared. Right-to-left differences in some brain regions of patients with RTT suggest that neuronal connectivity may have become restricted before development of specific skills contributing to interhemispheric asymmetry in these patients.
The third significant observation is that of reduced FA in the anterior cingulate gyrus, which is particularly revealing in view of the characteristic mood and behavioral changes seen in patients with RTT. In a similar setting, changes in the anterior cingulate gyrus and other components of the limbic system are also reported in schizophrenic28 and autistic29 patients. In addition, the posterior cingulate gyrus showed hemispheric asymmetry in the patients, which was not present in the control subjects. Larger studies are required, however, to investigate the correlation between severe behavioral and emotional problems in patients with RTT and limbic system components.
Volumetric MR imaging studies in RTT have shown a significantly reduced cerebral volume affecting both gray and white matter.7,30,31 In patients with RTT, MR imaging spectroscopy has shown significant decreases in average concentrations of N-acetylaspartate in both gray and white matter of the frontal and parietal lobes and insula compared with control subjects, demonstrating axonal involvement in RTT.24,23 Our in vivo observation confirms diffuse involvement of white matter tracts with areas of selective vulnerability in the limbic system components.
Longitudinal evaluations and studies in older subjects are needed for better understanding of changes in FA and its relationship to motor regression, and loss of speech noted in the older girls with RTT. Increased sample size will also allow better correlation of function to FA measurements. Improved technology with use of 3T or higher MR imaging modalities may provide better definition of tracts.
Clinical variability is currently assessed by neurologic examinations and mutation type. DTI studies would add specificity to these observations and help to understand the effects of individual mutations whose clinical effects are modified by age and X-inactivation in female patients. Therapeutic benefits may also be ascertained by their effects on neuronal tracts, either as improvement or lack of progressive reduction in FA.
Acknowledgments
We thank Terri Brawner and Kathleen Kahl for their technical support in acquiring the MR imaging scans as well as Carolyn Gillen for nursing assistance during the MR imaging examinations.
Footnotes
Supported by National Institute for Child Health and Human Development grant P01-HD-24448; National Institute on Aging grant AG-20012; National Center for Research Resources grant RR-15241; and Johns Hopkins Institute for Clinical and Translational Research grant UL1RR025005.
Indicates open access to non-subscribers at www.ajnr.org
References
- Received February 19, 2009.
- Accepted after revision June 18, 2009.
- Copyright © American Society of Neuroradiology