Abstract
BACKGROUND AND PURPOSE: Anosmia or hyposmia, often accompanied by changes in taste, is recognized as a common symptom that can assist in the diagnosis of coronavirus disease 2019 (COVID-19). The pathogenesis of olfactory dysfunction in COVID-19 is not yet fully understood. MR imaging represents a useful anatomic imaging method for the evaluation of olfactory dysfunction associated with varying etiologies, including viral infection, trauma, and neurodegenerative processes. This case-control study was conducted to compare quantitative measurements of olfactory anatomic structures between patients diagnosed with COVID-19 associated with persistent olfactory dysfunction and healthy controls.
MATERIALS AND METHODS: This study has a retrospective design. Cranial MR imaging was performed on all participants in both the patient and control groups. The bilateral olfactory bulb volume, olfactory tract length, and olfactory sulcus depth were measured in all patients.
RESULTS: A total of 116 people aged 18–60 years, including 36 patients diagnosed with COVID-19 and 80 controls, were included in the study. All measured values were compared between the patient and control groups. The right, left, and total olfactory bulb volume values were significantly lower in the patient group than in the control group. The patient group also had significantly lower right and left olfactory sulcus depth and olfactory tract length values compared with those in the control group.
CONCLUSIONS: MR imaging findings can be used to demonstrate olfactory injury in patients with COVID-19. The olfactory pathway may represent an alternative route for virus entry into the central nervous system.
ABBREVIATIONS:
- COVID-19
- coronavirus disease 2019
- CoV
- human coronavirus
- OB
- olfactory bulb
- OBV
- olfactory bulb volume
- OD
- olfactory dysfunction
- OSD
- olfactory sulcus depth
- OT
- olfactory tract
- OTL
- olfactory tract length
- SARS
- Severe Acute Respiratory Syndrome
Coronavirus disease 2019 (COVID-19) was first reported in Wuhan, China, in December 2019. Although fever, cough, and shortness of breath were initially considered to be the predominant symptoms, other unusual symptoms, including smell and taste disorders, are receiving increasing attention.1
Growing evidence indicates that neurotropism is a common feature of human coronaviruses (CoVs),2 and the virus has been detected in the CSF of patients with Severe Acute Respiratory Syndrome CoV (SARS-CoV) infections.3 SARS-CoV-2 has also been suggested to have neuroinvasive potential (eg, in the brain stem), which might be partially responsible for respiratory failure among infected patients.4 COVID-19 has been associated with a variety of CNS complications, including ischemic infarction, intracranial hemorrhage, acute hemorrhagic necrotizing encephalopathy, cerebral venous thrombosis, posterior reversible encephalopathy syndrome, and widespread leukoencephalopathy with microhemorrhage.5,6 Angiotensin-converting enzyme 2 serves as a functional receptor for SARS-CoV-2, and its expression and distribution throughout the nervous system suggest that SARS-CoV-2 might be directly or indirectly responsible for neurologic symptoms. The olfactory pathway has also been suggested to serve as an alternative pathway for viral entry into the CNS.7,8
During COVID-19 infections, chemosensory symptoms, such as olfactory dysfunction (OD), may occur as a form of viral prodroma or may present concurrently with the development of other disease symptoms.9 Anosmia or hyposmia, often accompanied by changes in taste, is now recognized as a common symptom that can assist in the diagnosis of COVID-19. However, the mechanism underlying these symptoms, which usually lasts for several weeks, has not yet been fully elucidated. Although the presence of OD in patients with COVID-19 was previously considered an unexpected symptom, the increasing number of patients with COVID-19 who present with OD has resulted in this symptom becoming a diagnostic criterion. Studies have reported taste and olfactory disorders at a rate of up to 88%, especially among mild and moderate cases.10 In a study examining 114 patients with anosmia, 98% recovered olfactory function within 4 weeks.11 Conversely, in another study of 1480 patients with COVID-19, OD was detected in 68% of the patients and was irreversible in 26% of those.12
The olfactory clefts are 2 narrow, vertical passages located in the upper part of the nasal cavity, forming an essential pathway for odor molecules in the air to reach the olfactory mucosa. Sensorial olfactory neurons arising from the olfactory mucosa pass through the cribriform lamina and form the olfactory bulb (OB), the terminal part.13 The OB is an ovoid, long, flat neural structure connected to the brain by the olfactory tract (OT). The OB and OT are found in the olfactory sulcus on the lower face of the frontal lobe.14 Projections arising from olfactory neural structures connect to the piriform cortex, amygdala, orbitofrontal cortex, thalamus, and insula.15
The standard measurements of the olfactory system with MR images were developed by Yousem et al15 in 1998. The absence or hypoplasia of the OB and OT seen on MR imaging has been associated with OD.16,17 Olfactory sulcus depth (OSD) is another parameter in the assessment of OD. Many diseases, including Parkinson, Behcet, and Alzheimer are associated with decreased OSD and reduction of the sense of smell.18 Many later studies have shown MR imaging to be a reliable method for evaluating the olfactory system.19,20 MR imaging–based evaluations of the olfactory structures are a useful anatomic imaging method for evaluating OD associated with a variety of causes, including viral infection, trauma, and neurodegenerative processes.21 MR imaging can be used to discriminate among the various etiologies that can cause OD, such as sinonasal and neurodegenerative diseases, and can predict OD prognosis.22 In adults, the OB can be easily identified by conventional MR imaging of the anterior cranial fossa, located above the cribriform plate and just below the olfactory sulcus.17,23 The total OB volume (OBV) can be obtained by calculating the sum of the right and left OBVs.24
The aim of the current study was to determine whether structural damage to the olfactory anatomic pathways could be detected in patients with COVID-19 with persistent OD by measuring the OBV, OT length (OTL), and OSD using MR imaging.
MATERIALS AND METHODS
The study was conducted in accordance with the tenets of the Declaration of Helsinki after obtaining approval from the ethics committee of Adiyaman University Faculty of Medicine. Informed consent was not obtained because the study had a retrospective design. There are no publicly stored data sets associated with this article; data are available on request from the corresponding author.
This case-control study was conducted to perform quantitative measurements of olfactory anatomic structures in patients diagnosed with COVID-19 who present with persistent OD and to compare them with those measurements from control individuals. The patient group is composed of people 18–60 years of age who had a positive COVID-19 diagnosis confirmed by positive findings on a polymerase chain reaction test on a nasopharyngeal swab sample taken due to the presentation of initial COVID-19 symptoms and who presented to the neurology outpatient clinic of Adiyaman Education and Research Hospital, due to OD. The control group consisted of individuals who presented to the neurology outpatient clinic for different, nondegenerative reasons (tension headache, tinnitus, and so forth) who had no history of COVID-19 or OD symptoms and underwent a cranial MR imaging examination and were reported to have normal MR imaging findings. The MR imaging examination of the patients with COVID-19 was performed within 2–8 weeks after the initial diagnosis made by polymerase chain reaction. The following patients were excluded from the study: those with systemic and endocrine diseases, such as chronic renal failure, chronic liver disease, rheumatologic disease, thyroid dysfunction, hypertension, and diabetes; those with B12 deficiency; those with neurodegenerative diseases, such as Parkinson disease, multiple sclerosis, and Alzheimer disease; those with a history of head trauma; and pregnant women. In addition, patients with sinonasal diseases, such as allergic rhinitis and chronic sinusitis, were excluded (Fig 1).
CONSORT flow diagram. HT indicates hypertension; DM, diabetes mellitus; TD, thyroid dysfunction; PD, Parkinson disease.
MR Imaging Protocol
All MR imaging was performed on a Signa Explorer 1.5T MR imaging machine (GE Healthcare) with a 16-channel head coil. MR images were used for volumetric and morphometric measurements. Coronal FIESTA with phase cycling (FIESTA-C) 3D T2-/T1-weighted images were obtained using the following protocol: TR = 7.6 ms; TE = 2.7 ms; FOV = 170 × 170 mm; number of excitations = 4; thickness = 1.6 mm; gap = 0 mm; number of sections = 1024; matrix = 300 × 300 mm.
Image Analysis
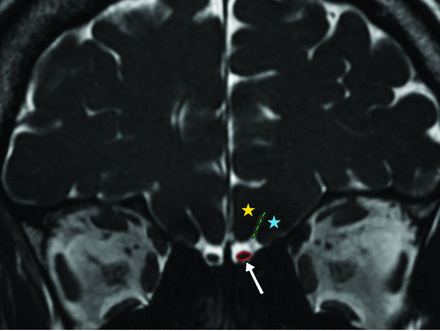
Bilateral OBV, OTL, and OSD measurements were performed in all participants. The OB was observed as a hypointense, ovoid structure surrounded by hyperintense CSF on a coronal FIESTA-C series and sections obtained at a right angle to the cribriform plate (Figs 2 and 3). Volumetric measurements were obtained by manual segmentation based on the contour stack principle on a 3D workstation. OBV was calculated in cubic millimeters. The OTL measurement, recorded in millimeters, was performed on multiplanar reconstruction of 3D FIESTA-C sagittal images at the section where the entire nerve trace was best visualized (Fig 4). The OSD was measured on coronal FIESTA-C 3D images by drawing a line tangential to the lower boundaries of the gyrus rectus and medial orbital gyrus and measuring the deepest point between these gyri and recorded in millimeters. All volumetric and length analyses were performed by 2 radiologists with at least 15 years of professional experience in head and neck radiology, and the measurements made by both radiologists were averaged.
Illustration of the olfactory system on coronal plane MR imaging. The medial orbital gyrus (red area), gyrus rectus (green area), olfactory sulcus (blue area), and olfactory bulb (brown area) are shown.
Coronal 3D-FIESTA-C MR image of a 41-year-old man showing the right and left olfactory bulbs as hypointense ovoid structures (arrow). The olfactory sulcus (green dashed lines) is seen as a hyperintense line between the medial orbital gyrus (blue star) and gyrus rectus (yellow star). Note the hyperintense CSF surrounding the OBs.
Sagittal multiplanar reconstruction of 3D-FIESTA-C MR imaging of a 27-year-old man showing the left olfactory tract (crosshairs).
Statistical Analysis
Statistical analyses were performed using SPSS, Version 22.0 (IBM). The compliance of all variables with a normal distribution was examined graphically using histograms and evaluated using the Shapiro-Wilk test. Descriptive statistics are presented as the mean (SD), median, and minimum–maximum values. Numeric variables were compared between groups using the independent t test because the data were found to be normally distributed. Categoric variables were compared using the Pearson χ2 test. The Mann-Whitney U test was used when comparing skewed variables between groups. A P value < .05 was considered significant. Interobserver agreement in OBV, OSD, and OTL measurements was evaluated using Bland-Altman graphics. The average difference and 95% limits of agreement (mean difference ± 1.96 SDs) are specified.
RESULTS
A total of 116 individuals 18–60 years of age, including 36 diagnosed with COVID-19 and 80 controls, were included in this study. The patient group included 19 (52.8%) women, and the control group included 44 (55%) women. The mean age of the patient group was 37.33 (SD, 7.38) years (range, 23–54 years) and that of the control group was 35.74 (SD, 8.38) years (range, 18–52 years). No significant difference was found between the mean ages of the patient and control groups (P = .329). The sociodemographic characteristics of the patient and control groups are summarized in Table 1.
Sex distribution and mean ages of the control and patient groups
In addition to typical COVID-19 symptoms and OD, 19 patients had persistent headaches, 6 had dizziness, and 8 experienced impaired taste. Sinonasal symptoms, such as nasal obstruction, were not detected in any of the patients included in the study. Nine patients had sudden-onset and isolated OD, whereas 13 experienced OD followed by the onset of COVID-19-related symptoms. Fourteen patients developed OD during the course of the COVID-19 infection. The chest CT images of 2 patients revealed pulmonary involvement. The clinical characteristics of the patients are summarized in Table 2.
Demographic and clinical features of patients
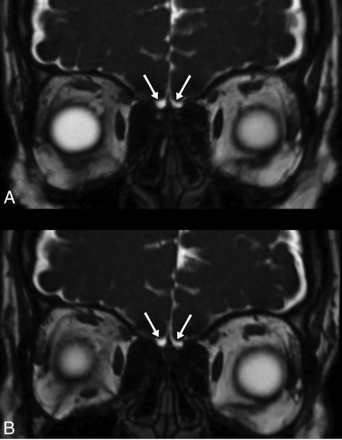
In 4 patients, the OBs were observed as bilateral points or lines in the coronal plane of MR images and were considered atrophic, with no changes observed for 1 patient on imaging at follow-up 3 months later (Fig 5). The measured values were compared between the patient and control groups. The right, left, and total OBV values were significantly lower in the patient group (41.57 [SD, 16.96], 40.76 [SD, 15.93], and 82.34 [SD, 31.29] mm3, respectively) compared with the control group (66.12 [SD, 16.86], 65.38 [SD, 18.80], and 131.50 [SD, 32.27] mm3, respectively; P < .001, P < .001, and P < .001). The right and left OTL values were significantly lower in the patient group (11.08 [SD, 2.18] and 11.24 [SD, 2.57] mm, respectively) compared with the control group (12.85 [SD, 2.14] and 12.80 [SD, 2.60] mm, respectively; P < .001 and P = .003). The right and left OSD values were also significantly lower in the patient group (8.33 [SD, 1.65] and 8.64 [SD, 1.40] mm, respectively) compared with the control group (9.20 [SD, 1.64] and 9.29 [SD, 1.53] mm, respectively; P = .01 and P = .033; Table 3).
Coronal plane 3D-FIESTA-C MR images of a 33-year-old female patient with loss of smell who was proved to have COVID-19. The olfactory bulbs are seen as atrophic (A). The olfactory bulbs are still atrophic, though the loss of smell has improved, on MR image of the same patient 3 months later (B).
Average, SD, and P values of OBV, OTL, and OSD measurements of patient and control groups
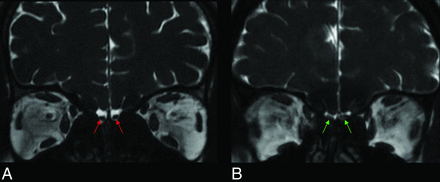
The key results are shown in Figs 6 and 7. In 7 cases (19.4%) in the patient group, focal intensity increased in the right OB in 4 patients, in the left OB in 2 patients, and in bilateral OBs in 1 patient (Fig 8). In the control group, focal intensity increased in the left OB in 1 individual. Normal and abnormal findings are shown side by side in Figs 9 and 10.
Boxplots of the right OBV, left OBV, and total OBV values in both patient and control groups.
Boxplots of the right OTL, left OTL, right OSD, and left OSD values in both patient and control groups.
Coronal 3D FIESTA-C MR images of a 26-year-old male from control group (A) and a 25-year-old female patient with COVID-19 anosmia (B). Normal and increased signal intensity in bilateral olfactory bulbs (red and green arrows, respectively).
Coronal 3D-FIESTA-C MR images of a 28-year-old man from the control group (A) and a 36-year-old female patient (B). Note normal and abnormal olfactory bulbs (red and green arrows, respectively) and olfactory sulci (red and green dashed lines, respectively).
Sagittal multiplanar reconstruction of 3D-FIESTA-C MR images of a 42-year-old woman from the control group (A) and a 36-year-old female patient (B) showing normal and abnormal olfactory tracts (red and green arrows, respectively).
DISCUSSION
The pathogenesis of OD associated with COVID-19 is not yet fully understood, but mechanisms that involve damage to the olfactory neural structures have been emphasized. In this study, we evaluated a group of patients who presented with COVID-19-associated OD, which persisted despite improvements in the typical symptoms associated with COVID-19. The findings of the study provided evidence suggesting that the olfactory anatomic structures might be affected by this disease.
Some features distinguish OD observed in COVID-19 from other postviral forms of anosmia. Postviral anosmia that develops following upper respiratory tract infections is generally associated with mucosal obstructions and nasal congestion, and its pathogenesis is predominantly conduction-type OD that develops secondary to impaired airflow.25 However, studies have not identified the significant coexistence of sinonasal symptoms with COVID-19 anosmia.26,27 In most of our COVID-19 positive patients, no pathological evidence was identified to suggest inflammation in any sinonasal sinuses, such as mucosal obstruction or nasal obstruction.
Different mechanisms are likely to cause OD pathogenesis during the course of COVID-19 infection. Although angiotensin-converting enzyme 2 receptors, which are the target molecules for SARS-CoV-2, are expressed by non-neuronal support cells in the olfactory epithelium, they are not expressed in neural cells,1 a feature that might explain the rapid recovery of olfactory function in many patients. However, CoVs have been reported to cause structural damage to olfactory neurons by directly invading olfactory sensory axons via the cribriform plate, and olfactory neuropathy has been detected in cases of SARS-CoV infections.2 Therefore, the neuroinvasive potential of SARS-CoV-2 has been hypothesized.1 The neurotrophic properties of SARS-CoV-2 may result in the destruction of sensory olfactory structures, and the retrograde viral invasion of olfactory pathways could explain the OD observed in COVID-19 infections to a certain extent. An example of olfactory neuroinvasion through a similar mechanism has been described for herpes simplex virus encephalitis.28 However, no such pathologic evidence is currently available to support a neuroinvasive mechanism during COVID-19 infection. The permanence of anosmia in some cases of COVID-19 supports the idea of neuronal damage. The atrophic appearance of the OBs of 4 patients was detected on MR images, and no change was observed on follow-up images after 3 months in 1 patient who has experienced persistent anosmia, consistent with this hypothesis.
Imaging of the olfactory nerve is not routinely performed, though OD has been reported with high frequency in patients with COVID-19 and represents a neurologic disease marker. An MR imaging examination can be useful for the evaluation of patients with anosmia and hyposmia, allowing for the elaborative visualization of olfactory anatomic structures and the quantitative and qualitative measurement of these structures. The literature indicates that available imaging of the olfactory structures in COVID-19-associated OD has been primarily reported in the form of case reports, with limited studies on this subject. To our knowledge, our study is the first to perform quantitative olfactory measurements, such as OBV and OSD, in patients with COVID-19 and to compare them against those in a control group. Bilateral OB atrophy was previously detected in the MR imaging examination (on coronal T2-weighted fat-suppressed images with 3- mm section thickness) of a patient with COVID-19 who experienced anosmia.29
In another case report, the MR imaging examination (on coronal 3D constructive interference in steady-state T2-weighted images) of a patient with anosmia with COVID-19 revealed the bilateral edematous and hyperintense appearance of the OB, but the OB returned to normal during the follow-up examination performed after 4 weeks.30 Tsivgoulis et al31 compared the height of the OB in cases of COVID-19 associated with OD with that in a control group (on coronal T2-weighted turbo spin-echo images with 2-mm section thickness) and reported a significant reduction in the patient group. Li et al32 showed (on coronal 3D T2-weighted turbo spin-echo images with 1-mm section thickness) a decrease in the right OBV and increased linear hyperintensities in the bilateral OBs of a patient with COVID-19 and anosmia. In a quantitative study of OB intensity (on coronal 3D-FLAIR sequence images with 2-mm section thickness) comparing patients with OD and normosmic COVID-19, the OB signal intensity in the OD group was significantly higher than that in the normosmic group.33
The results of our study are consistent with those of these previous studies. We found significant decreases in the values of all investigated MR imaging parameters (right OBV, left OBV, total OBV, right OTL, left OTL, right OSD, and left OSD) in the COVID-19 group compared with the control group. In addition, we found an increase in focal intensity in 7 patients in the patient group. We hypothesize that the presence of olfactory structural abnormalities detected on MR imaging in patients with COVID-19 may indicate olfactory neuropathy. In this respect, our study supports the idea that OD detected in patients with COVID-19 has a sensorineural origin, going beyond sinonasal involvement.
The presence of CNS complications, such as persistent headache and motor deficits, in patients with olfactory structural damage has led to the hypothesis that the virus uses the olfactory pathway as an entry route into the brain.34 In preclinical experiments performed in transgenic mice, neuronal losses and the identification of viral expression in various brain regions have been demonstrated following the nasal administration of CoVs.1 In a case report describing a patient with COVID-19, increased intensity was found in both the OB and the gyrus rectus. After 4 weeks, cortical hyperintensity completely disappeared on MR imaging, and OB hyperintensity decreased and became thinner.35 The PET scan of a patient with COVID-19 with normal MR imaging findings revealed hypoactivity in the left orbitofrontal cortex.36 In our study, we identified no structural abnormalities of the cranial structures in the MR imaging examinations of any patients with COVID-19. However, most patients described symptoms of persistent headache, impaired taste, and dizziness accompanying OD. The presence of CNS symptoms accompanying OD in these patients may indicate that the olfactory pathway serves as an alternative route for viral entry into the CNS. However, functional imaging, CSF, and cytologic examinations should be performed in larger patient groups to verify this hypothesis.
OD is likely a more common symptom of COVID-19 than has been reported. OD may exist in the background of disease symptomatology and may be overlooked. Patients may not be able to perceive unilateral anosmia because full unilateral anosmia can only be detected by a thorough physical examination. Therefore, OD should be considered as a potential symptom for the identification and isolation of infected patients to control the spread of the pandemic.
This study has certain limitations. The number of patients participating in the study was relatively small. In addition, the decision not to evaluate patients with severe pulmonary and systemic infections and long disease durations may create concerns related to possible patient selection bias. Another limitation is that patients’ self-reported OD was accepted without performing objective smell-identification tests.
CONCLUSIONS
Clinicians should not overlook OD as a potential indicator for disease diagnosis, the determination of prognosis, pandemic control, and patient isolation. MR imaging can allow the diagnosis and prognosis prediction in patients with OD. MR imaging findings can be used to demonstrate olfactory injury in patients with COVID-19. We believe that studies comparing the initial and follow-up MR images performed in a larger number of patients are necessary to fully elucidate the mechanisms underlying SARS-Cov-2-induced OD. Future functional imaging and tractography studies will continue to shed light on the subject.
Acknowledgments
The authors are grateful to Ece Baykan for her contribution to the design of the figures and illustrations and to MRI technician Kasim Tepe and Uǧur Erol for their contributions to the creation of the MR imaging protocol.
Footnotes
The institution from which the work originated: Adiyaman University Faculty of Medicine, Yunus Emre Mahallesi 1164 Sokak No:13 02200 Merkez/Adıyaman/Turkey.
Disclosures: Sukru Mehmet Erturk—UNRELATED: Payment for Lectures Including Service on Speakers Bureaus: Bayer AG, Siemens.* *Money paid to the institution.
The following are the authors’ contributions: data collection: A.H.B, E.A; study design: A.H.B, E.A, E.Ay; manuscript writing: E.A; statistical analysis: S.S; figures and illustration: A.H.B; review and final editing: A.H.B, E.A, S.M.E. All authors contributed to the elaboration, critical revision, and review of intellectual content. All authors read and approved the final manuscript.
Indicates open access to non-subscribers at www.ajnr.org
References
- Received March 31, 2021.
- Accepted after revision August 1, 2021.
- © 2021 by American Journal of Neuroradiology