Abstract
BACKGROUND AND PURPOSE: Interictal FDG-PET scans are a routine diagnostic technique for the identification of epileptogenic foci in the presurgical work-up of medically refractory pediatric epilepsy. With the advent of PET/MR imaging, it has become possible to simultaneously acquire FDG-PET and arterial spin-labeling perfusion data. The objective of this study was to evaluate whether the incorporation of arterial spin-labeling data with interictal FDG-PET could improve the diagnostic performance metrics of FDG-PET for identification of epileptogenic foci.
MATERIALS AND METHODS: Forty-five pediatric patients with a mean age of 10.8 years were retrospectively included in this study. These patients all underwent PET/MR imaging to diagnose suspected focal epilepsy.
RESULTS: When compared to interpretations of interictal FDG findings alone, FDG combined with arterial spin-labeling findings resulted in significantly decreased sensitivity (0.64 versus 0.52, P = .02), significantly increased specificity (0.50 versus 0.75, P = .04), and an increased positive predictive value (0.59 versus 0.75). The decreased sensitivity was found to be primarily driven by patients with extratemporal lobe epilepsy, as a subgroup analysis showed decreased sensitivity for patients with extratemporal epilepsy (0.52 versus 0.38, P = .04), but not for temporal epilepsy (0.83 versus 0.75, P = .16). Additionally, substantial agreement between focal FDG hypometabolism and arterial spin-labeling hypoperfusion was demonstrated with the Cohen κ (0.70, P < .01).
CONCLUSIONS: These findings suggest that simultaneously acquired interictal FDG-PET and arterial spin-labeling data can improve the diagnosis of epileptogenic foci, especially in the setting of temporal lobe epilepsy where they improve specificity and positive predictive value, with preservation of sensitivity.
ABBREVIATIONS:
- ASL
- arterial spin-labeling
- MEG
- magnetoencephalography
- Tc99m-ECD
- technetium Tc99m ethyl cysteinate dimmer
- sEEG
- stereotactic electroencephalogram
- vEEG
- video electroencephalogram
Pediatric epilepsy is the most common neurologic disease affecting children, with an estimated prevalence of 0.69%.1 Of these patients, a substantial proportion are affected by focal epilepsy, with 1 study finding this diagnosis in >50% of newly diagnosed patients based on clinical and diagnostic evidence.2 Structural abnormalities typically underlie focal epilepsy, including developmental anomalies such as cortical dysplasia and polymicrogyria or acquired lesions like mesial temporal sclerosis and hypothalamic hamartomas. Approximately 20%–25% of pediatric patients with epilepsy will be refractory to medical management, typically defined as failure to achieve sustained seizure remission following 2 antiepileptic regimens.3⇓-5 This often prompts work-ups for focal epilepsy with numerous diagnostic modalities, including continuous video electroencephalogram (vEEG), magnetoencephalography (MEG), MR imaging, and FDG-PET, with the results of these studies informing electrode placement for the more invasive stereotactic EEG (sEEG).
Interictal FDG-PET plays an integral role in finding candidate regions for seizure onset due to the expected hypometabolism these foci exhibit in the interictal state. It has been found to be an accurate and cost-effective technique for this purpose, especially in cases of negative or equivocal EEG and MR imaging results.6,7 Its utility for temporal lobe epilepsy is especially high, with a recent meta-analysis by Niu et al8 demonstrating FDG-PET concordance rates with a reference standard of 0.79 for temporal epilepsy, but only 0.66 for extratemporal epilepsy. With the advent of PET/MR imaging, it has become possible to simultaneously acquire interictal FDG-PET data and coregistered high-resolution anatomic MR images. This feature has clear benefits with respect to more precise localization of focal hypometabolism and improved correlation of FDG uptake with underlying structural abnormalities. PET/MR imaging has also enabled correlation of metabolism with other relevant physiologic parameters that can be interrogated with MR imaging, most notably cerebral perfusion.
The effect of focal epilepsy on cerebral perfusion is analogous to that of FDG-PET, with increased focal perfusion during a seizure and decreased focal perfusion during the interictal period. This relationship has been well-characterized with ictal and interictal SPECT with perfusion radiotracers such as technetium Tc99m ethyl cysteinate dimmer (Tc99m-ECD).9,10 PET/MR imaging allows for an efficient and noncontrast assessment of cerebral perfusion simultaneous with PET with arterial spin-labeling (ASL) sequences.11,12 In the simplest form of ASL, a nonspatially selective 180° radiofrequency pulse is applied across the FOV, followed by a spatially selective 180° radiofrequency pulse overlying water protons, which serve as a proxy for blood, in the carotid and vertebral arteries. Precisely timing the onset of the subsequent pulse sequence, which is often a gradient-echo or fast spin-echo sequence, allows measurement of the magnetic relaxation of these flowing water protons in isolation from the stationary background. A delay in signal acquisition is then used to account for the time required for these water protons to traverse the intracranial vasculature and perfuse the brain parenchyma. The result is an image with signal in proportion to the relaxation of water protons (ie, blood) at each voxel, and thus cerebral perfusion.
Numerous studies have sought to evaluate the effectiveness of ASL techniques in the diagnosis of focal epilepsy during interictal imaging. A recent meta-analysis by Zeng et al13 analyzed 6 such studies with 174 patients and calculated a pooled sensitivity and specificity of 0.74 and 0.35 for ASL in the localization of epileptic foci. These mediocre diagnostic performance measures imply that ASL is likely best applied as an adjunct diagnostic test to other existing well-validated better performing modalities. From this perspective, the current study sought to compare the performance of interictal FDG-PET combined with ASL versus FDG-PET alone in the diagnosis of pediatric focal epilepsy, with the hypothesis that the addition of ASL would improve diagnostic benchmarks.
MATERIALS AND METHODS
Approval for this study was granted by the Stanford University institutional review board. We retrospectively reviewed the medical charts of patients who underwent interictal FDG-PET/MR imaging from January 2018 to June 2020. Inclusion criteria were patients younger than 18 years of age, clinically suspected focal epilepsy, and previously performed sEEG or continuous vEEG. Patients found to have artifactual nondiagnostic ASL sequences, most commonly related to arterial transit artifacts, were excluded from the study.
The FDG-PET and MR imaging were performed with a 3T Signa PET/MR scanner (GE Healthcare) and a 24-channel head coil. A volumetric gradient-echo T1 sequence (BRAVO; GE Healthcare) was acquired for anatomic reference with the following parameters: TR = 8 ms, TE = 3 ms, flip angle = 12°, acquisition matrix = 512 × 512, FOV = 240 × 240 mm, section thickness = 1 mm. A single postlabeling delay pseudocontinuous ASL technique was used for assessment of cerebral blood flow and, notably, is the ASL technique recommended for routine clinical use by the International Society for Magnetic Resonance in Medicine Perfusion Study Group and the European ASL in Dementia consortium.14 Additional ASL sequence parameters included the following: TR = 4685 ms, TE = 11 ms, flip angle = 111°, acquisition matrix = 512 × 512, FOV = 240 × 240 mm, section thickness = 4 mm. Numerous other MR imaging pulse sequences were acquired during these examinations but were not reviewed as part of this study.
Patients were instructed to fast a minimum of 4 hours before their examination. Baseline blood glucose levels were acquired to ensure values were <200 mg/dL. [18F] FDG–administered activities were based on patient weight per the 2016 North American Consensus Guidelines for Pediatric Administered Radiopharmaceutical Activities and ranged from 1.12 to 5.1 mCi, with a mean activity of 3.54 mCi.15 Before and following radiotracer administration, patients were placed in a quiet, dark room and instructed to avoid speaking, movement, and other stimulating activities when possible. The latency between radiotracer injection and image acquisition was approximately 60 minutes. PET imaging was performed in 1 bed position overlying the head, with 3D and TOF acquisitions, using a zero TE pulse sequence for PET attenuation correction. Other relevant parameters were as follows: 10-minute static image acquisition, acquisition matrix = 256 × 256, and diagonal FOV = 30 cm. The young age of many of the patients necessitated the use of general anesthesia during imaging to reduce motion artifacts. In these patients, anesthesia induction with IV propofol occurred 30 minutes following radiotracer injection.
Three independent readers interpreted both the interictal FDG-PET and ASL results for each patient, including 2 attending radiologists board-certified by the American Board of Radiology and American Board of Nuclear Medicine and a senior resident board-eligible by the American Board of Radiology and American Board of Nuclear Medicine with a mean of 8.3 years reading brain FDG-PET examinations. These readers were blinded to the patient’s clinical epileptic semiology and EEG findings. To more closely mimic clinical practice, we performed all assessments qualitatively. Interpretations were performed with MIMneuro™ (MIM Software Inc.). The FDG-PET data were separately coregistered to both a simultaneously acquired T1 anatomic sequence and an ASL sequence. Readers first analyzed the coregistered FDG-PET/T1 imaging to identify locations of relatively decreased FDG uptake. Once these locations were identified, the readers then correlated these sites on the coregistered FDG-PET/ASL imaging to determine whether there was coexisting decreased signal on ASL. From these observations, interpretations for FDG alone were defined as positive if focal hypometabolism was present on the PET imaging, regardless of the ASL findings. In contrast, interpretations for FDG combined with ASL were defined as positive only when both focal hypometabolism was present on PET imaging and focal hypoperfusion was present on ASL imaging. Discordant interpretations between the readers were resolved through collective discussion among the readers to yield a definitive consensus interpretation.
Statistical analysis was performed with NCSS Statistical Software (NCSS). Sensitivity and specificity comparisons between FDG alone and FDG combined with ASL were performed using the Nam-Blackwelder method for analysis of marginal probabilities in paired samples.16 This statistical test required separately coding FDG-only and FDG with ASL results for each patient as either true-positive, true-negative, false-positive, or false-negative. The criterion standard for this coding was based on sEEG results or alternatively vEEG results when sEEG was not performed. Additionally, accuracy, positive predictive value, and negative predictive value were calculated for FDG-only and FDG combined with ASL but were intended for qualitative interpretation only in the absence of validated statistical methodologies for comparison. Subsequent analysis also included assessment of the agreement between the FDG and ASL findings with the use of the Cohen κ.
RESULTS
Forty-five patients who met the inclusion and exclusion criteria for this study were retrospectively identified. The mean blood glucose level before FDG-PET/MR imaging was 91.3 mg/dL, and the mean interval between radiotracer injection and scanning was 56 minutes and 18 seconds. This cohort included 23 females and 22 males, with a mean age of 10.8 years, with an SD of 4.8 years. Seventeen of these patients had accessible sEEG reports, with the remainder only having undergone vEEG. Thirty-three patients were found to have epileptogenic foci on EEG. Among these patients, 11 temporal and 24 extratemporal foci were identified, with 2 patients having foci in both regions. Twelve patients underwent surgical resection or laser ablation based on the results of the EEG, PET/MR imaging, and other clinical data, with all demonstrating at least some improvement in their seizure frequencies.
The results of the FDG-only analysis identified 27 foci of decreased uptake, and the FDG combined with ASL analysis identified 20 foci of decreased uptake, with the breakdown by site of uptake further described in Table 1. Sample cases and their associated interpretations are illustrated in Figs 1 and 2. The sensitivity and specificity for the FDG-only analysis were 0.64 and 0.50, respectively. The sensitivity and specificity for the FDG combined with ASL analysis were 0.52 and 0.75, respectively. The difference between the sensitivities and specificities was found to be statistically significant, with a Nam test statistic of 2.0 (P = .02) for sensitivity and a Nam test statistic of −1.73 (P = .04) for specificity. Overall accuracy and the negative predictive values were similar between FDG-only and FDG combined with ASL, including 0.51 versus 0.53 and 0.38 versus 0.36, respectively. However, the positive predictive value was relatively increased with FDG combined with ASL compared with FDG-only (ie, 0.75 versus 0.59).
Patient diagnostic results
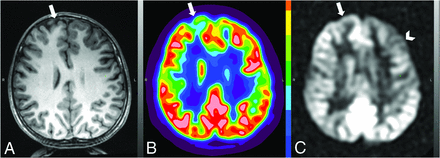
Concordant FDG-PET and ASL findings. A patient with focal epilepsy localized to the left precentral gyrus on sEEG with concordant findings on both FDG-PET and ASL. A, T1-weighted structural imaging for anatomic reference without an abnormality in the left precentral gyrus (arrow). B, FDG-PET image demonstrates focally decreased uptake in the left precentral gyrus (arrow) and more mildly decreased uptake in the broader left frontal lobe. C, Associated low signal on ASL in the left precentral gyrus (arrow) and more mildly decreased uptake in the broader left frontal lobe.
Discordant FDG-PET and ASL findings. A patient with focal epilepsy localized to the left insula on sEEG with discordant findings on FDG-PET and ASL. A, T1-weighted structural imaging for anatomic reference without an abnormality in the right superior frontal gyrus (arrow). B, FDG-PET image demonstrates focally decreased uptake in the right superior frontal gyrus (arrow). C, ASL image with absence of a correspondingly low signal in the right superior frontal gyrus (arrow) but with apparent low signal overlying the left frontal lobe (chevron), which is of unclear clinical significance.
Sensitivity was also separately calculated and compared in a subgroup analysis in which patients were divided into temporal and extratemporal lobe epilepsy groups based on their EEG findings. In patients with temporal lobe epilepsy, there was no significant difference in sensitivity with the use of FDG-only compared with FDG combined with ASL, 0.83 versus 0.75 (Nam test statistic, 1.0; P = .16). In contrast, for extratemporal lobe epilepsy, the sensitivity was significantly higher in FDG-only compared with FDG combined with ASL, 0.52 versus 0.38 (Nam test statistic, 1.73; P = .04) (Table 2).
Diagnostic comparison of FDG versus FDG and ASLa
In a final analysis, we sought to assess the agreement between interictal FDG and ASL findings. Findings were concordant for 20 examinations with positive findings, and 18 examinations with negative findings. For 7 examinations, FDG findings were positive, but ASL findings were negative. No examinations demonstrated a negative FDG finding and a positive ASL finding, which was an expected result based on the approach to interpretation discussed in the Materials and Methods section. Statistical analysis with the Cohen κ demonstrated substantial agreement between the FDG and ASL findings (κ = 0.70, P < .01) (Table 3).
FDG and ASL agreementa
DISCUSSION
The results of this study demonstrate the potential role that ASL may play as an adjunct technique in the diagnosis of focal epilepsy. Using both interictal FDG and ASL data during interpretations improved the specificity and positive predictive value for epileptogenic foci identification compared with FDG-only. However, this use was paired with a corresponding decrease in overall sensitivity compared with FDG-only. Previous studies have compared interictal FDG-PET and ASL findings, with results largely showing high concordance in the results of these techniques.17⇓-19 Boscolo Galazzo et al20 evaluated the results of brain FDG-PET/MR imaging in 20 patients with refractory focal epilepsy and demonstrated substantial agreement between FDG and ASL findings with a Cohen κ value of 0.72, which was comparable with the 0.70 value in this study. Shang et al21 reported complementary findings, with a high correlation between normalized standard uptake values for FDG-PET and ASL CBF values (Spearman rank correlation coefficient =0.59). Moreover, they demonstrated improved sensitivity and specificity for localization of seizure foci when FDG and ASL findings were combined. Notably, these latter findings required quantitative assessment of the FDG and ASL data, with the use of logistical regression modeling to identify FDG standard uptake values and ASL CBF cutoff values. This finding contrasts with the approach of the current study of an entirely qualitative evaluation of FDG and ASL data with only dichotomous outcome measures (ie, positive/negative). This method of analysis was chosen to more accurately simulate clinical practice, in which the qualitative visual assessment is the primary means of interpretation. Consequently, the findings of this study illustrate a practical method for clinicians to incorporate ASL imaging into their interpretations of interictal PET/MR imaging examinations. Specifically, ASL can be used to increase the confidence that a focus of FDG hypometabolism represents an epileptogenic focus but with the understanding that it comes with an expected cost of decreased sensitivity compared to interpretation with FDG-alone.
Furthermore, the sensitivity penalty imparted by incorporation of ASL data into interpretations appears to be most relevant in extratemporal as opposed to temporal epilepsy. It has been well-documented that interictal FDG-PET is more sensitive in localizing epileptogenic foci in temporal compared with extratemporal regions, with a study by Drzezga et al22 reporting 0.86–0.9 sensitivity for temporal lobe foci, and 0.33–0.38 sensitivity for extratemporal foci. These findings are corroborated by the current examination, with higher sensitivity for temporal lobe compared with extratemporal foci with FDG-only interpretations (0.83 versus 0.52) and also for FDG combined with ASL interpretations (0.75 versus 0.38). Moreover, the results also demonstrate that incorporation of ASL imaging into interictal FDG-PET interpretation significantly decreases sensitivity only for extratemporal foci (0.52 versus 0.38, P = .04) and not for temporal foci (0.83 versus 0.75, P = .16). The underlying etiology of this discrepancy is not entirely clear, but it may, in part, relate to a previously speculated mechanism whereby extratemporal seizure foci, especially those in the frontal lobe with its more robust intracerebral connectivity, propagate more quickly into neighboring regions, resulting in broader areas of milder hypometabolism or hypoperfusion in the case of ASL.23 As a consequence, these broader areas of FDG or ASL abnormalities may be more difficult for readers to identify. Regardless of the etiology, this finding allows clinicians to have increased confidence in ASL results in the temporal lobe as opposed to extratemporal regions when rendering their interpretations.
The high level of agreement between interictal FDG-PET and ASL findings for epileptogenic foci demonstrated in the current and previous studies also prompts consideration of ASL as a potential replacement for FDG-PET.17⇓-19 This would be highly beneficial in terms of reduced exposure to ionizing radiation, reduced cost, and decreased duration of general anesthesia in younger patients relating to shorter scan times. From recent pooled meta-analyses, sensitivities for interictal FDG-PET and ASL were calculated to be 0.66 and 0.74, respectively, and their specificities, 0.71 and 0.35, respectively.8,13 Although the sensitivities are similarly modest, FDG-PET has a notable advantage in terms of specificity. The low specificity for epileptogenic foci of ASL is overall indicative of a high false-positive rate. This matches our collective clinical experience with ASL as implemented at our institution, in that numerous areas of hypoperfusion are often identified on patient scans, which are of unclear clinical significance and in some cases may be artifactual. This phenomenon is well-demonstrated in a patient with discordant sEEG, FDG, and ASL findings as seen in Fig 2. To mitigate this issue, we designed the current study so that only foci with identified FDG hypometabolism would be analyzed on ASL, disregarding ASL signals in other areas of the brain. However, future investigations may benefit from emerging advances in ASL techniques, which have the potential to provide more reliable cerebral perfusion data. These notably include the development of competing methods to correct for individual patient variation in the arterial transit time of labeled water protons.24,25 Regardless, at present, ASL cerebral perfusion data should be viewed as supplemental to FDG-PET and cannot supplant the clinical value of FDG-PET in the diagnostic work-up of focal epilepsy.
Cerebral perfusion and cerebral metabolism are not wholly coupled processes in the setting of epilepsy.26 Prior research in temporal lobe epilepsy has demonstrated that FDG-PET often demonstrates multiple other extratemporal sites of hypometabolism, possibly related to cerebral diaschisis, that are often without correlates on modalities examining cerebral perfusion, including ASL.27 This finding may suggest that each technique is providing unique, and as demonstrated in this study, diagnostically beneficial data on focal epilepsy. As ASL gains further acceptance clinically, it may represent a quick, effective, and noncontrast method to assess cerebral perfusion, which can readily be added to most FDG-PET/MR imaging interictal protocols.
To our knowledge, this is the largest patient sample reported in the literature to have both FDG and ASL imaging analyzed in the identification of epileptogenic foci. Furthermore, only 3 prior studies have acquired these data simultaneously with the use of PET/MR imaging, a technique that serves to minimize the impact of temporal-related intrasubject variability on FDG and ASL findings. This study is also the first analysis performed exclusively in a pediatric population. Pathophysiologic differences exist between pediatric and adult epilepsy, which would reasonably be expected to impact structural and functional neuroimaging findings. When a discrete cause of seizures is present, cerebrovascular abnormalities and neoplasms are identified as the etiology in most adult epilepsy cases, whereas a broader array of pathologies are observed in children, such as perinatal insults, congenital malformations, genetic diseases, and inborn errors of metabolism.28⇓-30 However, despite these etiologic differences, the results of this investigation help to confirm that the close relationship between FDG-PET and ASL is similar in adult and pediatric focal epilepsy populations.
Limitations of this research include the retrospective nature of the analysis and limited generalizability of novel findings outside pediatric patients. The use of vEEG results as the reference standard in patients who did not undergo sEEG may have introduced some degree of bias in our results relating to its decreased accuracy relative to the latter method. Another potential source of bias that was inherent to the study design includes interpreters having knowledge of the FDG-PET findings before analyzing the ASL data. Finally, the necessity of general anesthesia with propofol in some patients may have impacted our results due to effects on cerebral metabolism and perfusion. However, propofol has, in some studies, been found to have the least impact on these physiologic parameters compared with other general anesthesia agents.31,32
CONCLUSIONS
This study serves to highlight the clinical application of ASL in the localization of epileptogenic foci in pediatric patients. When interpreted alongside simultaneously acquired interictal FDG-PET, ASL improves diagnosis of focal epilepsy, especially as it pertains to temporal lobe epilepsy where it improves specificity and PPV, while maintaining a similar level of sensitivity. With the increasing availability of PET/MR scanners, it may become possible to efficiently implement ASL imaging into routine interictal FDG-PET scans in the pursuit of improved patient care.
Footnotes
Disclosure forms provided by the authors are available with the full text and PDF of this article at www.ajnr.org.
References
- Received August 15, 2021.
- Accepted after revision December 6, 2021.
- © 2022 by American Journal of Neuroradiology