Abstract
Summary: Submicroscopic deletion of the terminal part of the short arm of chromosome 6, including 6p25, leads to developmental retardation, hearing impairment, ocular dysgenesis, and dysmorphic features. We diagnosed 3 patients referred because of white matter abnormalities of unknown origin. MR imaging showed multifocal areas of abnormal signal and enlarged perivascular spaces in the cerebral white matter that were stable during follow-up. Multifocal white matter abnormalities are most commonly seen in static, nonmetabolic encephalopathies, including chromosomal abnormalities.
There are many different known causes for leukoencephalopathies in children, both inherited and acquired. MR imaging patterns can be of great value in guiding the diagnostic process.1,2 Some MR imaging characteristics have been found predominantly in association with static, nonmetabolic encephalopathies2 (eg, those caused by congenital infections3,4 and chromosomal abnormalities5,6). In this article, we describe the MR imaging findings in 3 patients who were referred because of a leukoencephalopathy of unknown origin, with the 6p25 deletion syndrome.
Case Reports
Patient 1
Patient 1 was a girl, born in 1998 as the first child of healthy, unrelated parents. She had a mild psychomotor retardation and dysmorphic features, including hypertelorism, epicanthal folds, a low, wide nasal bridge, a small, short nose with anteverted nares, and crowded dentition. In addition, she had a short stature (height below 2.5 SD), normal head circumference, ophthalmologic abnormalities (hypermetropia, forme fruste of a mesodermal dysgenesis, and embryotoxon posterior [anterior displacement and prominence of the Schwalbe’s line]), cardiac abnormalities (bicuspid aortic valve and valvular aorta stenosis), and 1 kidney. Laboratory investigations, including extensive metabolic studies, chromosomal analysis, and a polymerase chain reaction (PCR) for cytomegalovirus (CMV) DNA on the Guthrie card were unrevealing.
MR imaging of the brain at of 2, 3, and 4 years of age revealed multiple small cerebral white matter lesions on T1- and T2-weighted images. In addition, a multitude of radiating stripes were seen within the cerebral white matter, which had a low signal intensity on fluid-attenuated inversion recovery (FLAIR) images, consistent with diffusely enlarged perivascular spaces. The cerebellar vermis was small, and the fourth ventricle was dilated. The abnormalities did not change with time.
Patient 2
Patient 2 was a boy, born in 2002 as the brother of patient 1. He was more severely developmentally delayed than his sister and displayed autistiform behavior. He had a short stature and dysmorphic features, including hypertelorism, blepharophimosis, long, thick eyelashes, a broad, low nasal bridge, a small, short nose with anteverted nares, a broad, tent-shaped mouth, a small mandibula, a short, broad neck, and bilateral ear tags. He had a sensorineural hearing loss. His head circumference was normal. Cardiac investigation revealed a bicuspid aorta valve, but no stenosis. Ophthalmologic examination at 2 years of age showed bilateral embryotoxon posterior, but no further anterior eye chamber abnormalities. His karyotype was normal.
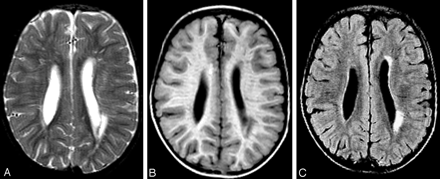
MR imaging of the brain in the neonatal period and at 2 years of age showed slightly dilated lateral ventricles (Fig 1). In addition, there were multiple periventricular white matter lesions on T1- and T2-weighted images (Fig 1A, -B), a multitude of radiating stripes with high signal intensity on T2-weighted images (Fig 1A), and a low signal intensity on T1-weighted (Fig 1B) and FLAIR images (Fig 1C), consistent with enlarged perivascular spaces within the cerebral white matter.
MR imaging of patient 2 at 2 years of age. On the axial T2-weighted (A), T1-weighted (B), and fluid-attenuated inversion recovery (FLAIR) (C) images, multifocal cerebral white matter lesions are visible, many presenting as thin radiating stripes suggesting increased perivascular spaces, some presenting as larger foci. The lateral ventricles are mildly dilated.
For further diagnostic work-up, multiplex amplifiable probe hybridization (a high-throughput molecular technique to detect submicroscopic chromosomal deletions and duplications) was performed. The probes used in this analysis included all subtelomeric and acrocentric regions and regions related to microdeletion syndromes. This assay revealed a subtelomeric deletion of chromosome 6p and a subtelomeric duplication of chromosome 20q. Additional fluorescence in situ hybridization (FISH) analysis by using probes specific for the subtelomeric region of the short arm of chromosome 6 and the long arm of chromosome 20 confirmed the suspected unbalanced translocation with a deletion of chromosome 6p and a duplication of chromosome 20q [46,XY,der(6)t(6;20)(p25.1;q13.3)]. The same abnormality was found in his sister, patient 1. The mother carried a balanced translocation 46,XX,t(6;20)(p25.1;q13.3); the father had normal chromosomes.
Patient 3
Patient 3 was a girl, born in 2002, as the first child of healthy unrelated parents. A systolic murmur was found soon after birth, but cardiologic investigation including ECG and cardiac sonography revealed no abnormalities. She had a delayed development and dysmorphic features with down-slanting palpebral fissures, a flat, broad nasal bridge, a short nose, and low-set ears. Ophthalmologic examination revealed hypertelorism, hypermetropia, papillary dysplasia, a persistent pupillary membrane, and coloboma of the left iris. She was diagnosed with a sensorineural hearing loss. She had normal height and head circumference. Her diagnostic work-up, including extensive metabolic studies, chromosomal analysis, and a PCR for CMV DNA on the Guthrie card was unrevealing.
MR imaging at 1 and 2 years of age demonstrated slightly dilated lateral ventricles. There were more extensive multifocal and partially confluent areas of high signal intensity on both T2-weighted (Fig 2A) and FLAIR images (Fig 2B), involving the periventricular, deep, and subcortical cerebral white matter. In addition, many enlarged perivascular spaces were seen, indicated by radiating stripes of low signal intensity on coronal FLAIR images (Fig 2B) and dots of low signal intensity on parasagittal T1-weighted images (Fig 2C). The corpus callosum was short (Fig 2D). The cerebellar vermis was small, and the fourth ventricle was dilated (Fig 2D). The MR imaging abnormalities did not show progression with time.
MR imaging of patient 3 at 2 years of age. The axial T2-weighted (A) and coronal fluid-attenuated inversion recovery (FLAIR) (B) image show that in this patient, the multifocal white matter abnormalities are more extensive with larger areas of abnormal signal intensity than those in patient 2. In addition, there are radiating stripes compatible with increased perivascular spaces, readily seen on coronal FLAIR images (B). The sagittal T1-weighted images confirm the presence of many enlarged perivascular spaces (C). The corpus callosum is short; the rostrum is missing (D). The cerebellar vermis is small (D). The lateral (A, -B) and fourth ventricles (D) are mildly dilated.
FISH with DNA probes specific for the subtelomeric region of the short arm of chromosome 6 (6p25) was performed because of the striking similarities in facial and MR imaging features in patients 1 and 2, and indeed a deletion of this locus was found. The deletion was not present in the parents.
Discussion
A submicroscopic deletion of the terminal part of the short arm of chromosome 6 is associated with a multitude of developmental anomalies.7–10 Psychomotor retardation, hearing impairment, and ocular dysgenesis are almost invariably present. The ocular abnormalities usually consist of developmental anomalies of the anterior chamber of the eye, including corneal opacities, iris coloboma, and hypoplasia of the iris with adherent iris strands to the peripheral cornea (Rieger anomaly). Hypermetropia may be present. The patients also have dysmorphic features, including hypertelorism, down-slanting palpebral fissures, small nose, low nasal bridge, tented mouth, and a short neck. Other features that have been described include skeletal, renal, and cardiac malformations. The encephalopathy is static, and its substrate is unknown.
Neuroimaging findings have been described only in occasional patients with the 6p25 deletion syndrome.7–10 The most common abnormalities in the small series of Davies et al7 and Gould et al8 were hydrocephalus and atrophy, both referring to enlarged CSF spaces. Head circumference, however, was reported to be normal, and shunt surgery was not necessary in any of the patients, suggesting mild cerebral hypoplasia rather than hydro-cephalus. In addition, a thin corpus callosum and a small cerebellar vermis or Dandy-Walker-like malformation have been reported.7,8,10 Many of the patients were reported to have normal findings on CT,7,8 but CT may fail to reveal subtle abnormalities. White matter abnormalities have been reported in only 2 patients. Davies et al7 described the brain MR imaging of 1 patient as revealing gliosis of the cerebral white matter with increased perivascular spaces, but the images were not shown. The patient reported by Chen et al9 had extensive signal intensity abnormalities in the cerebral white matter, partially multifocal, partially more confluent, but enlarged perivascular spaces were not mentioned.
In our 3 patients, we found static, multifocal cerebral white matter lesions in combination with diffusely enlarged perivascular spaces. Less striking abnormalities, which were variably present, included mildly dilated lateral ventricles, small cerebellar vermis, and a short corpus callosum.
The pattern of imaging abnormalities in our patients is reminiscent of that seen in CMV infection.3,4 However, it lacks several features that are particularly suggestive of congenital CMV infection such as a parietal predominance of the white matter abnormalities and anterior temporal abnormalities, including swelling and signal intensity abnormality of the temporal white matter, anterior temporal cysts, and dilated inferior horns of the lateral ventricles.4 Congenital CMV infection was excluded in our patients. The pattern of imaging abnormalities in the present patients is not really suggestive of congenital infection by Toxoplasma gondii or rubella.11,12 The latter congenital infections are characterized by tissue destruction of variable severity11,12 and not by the selective and often extensive white matter lesions without manifest tissue loss that is typically seen in CMV.4
The imaging findings in the patients could be considered reminiscent of periventricular leukomalacia (PVL), which is related to perinatal asphyxia and occurs most commonly in premature infants.13 Pregnancy, delivery (at term), and the perinatal period were unremarkable in our patients, and they did not show signs of cerebral paresis. Only 1 of the 3 MR imaging features diagnostic for PVL was present (periventricular white matter signal intensity abnormalities), whereas the other 2 features were not (loss of white matter volume, the depth of the sulci almost touching the ventricles; and scarring of the ventricular wall, leading to irregular deformation). All in all, the diagnosis of PVL is unlikely in our patients.
The possible nature of the white matter abnormalities in the 6p25 deletion syndrome is unclear. Considering that the white matter abnormalities are static and that the patients present with a range of other developmental anomalies, we believe that focal deficits in myelination and gliosis may underlie the signal intensity abnormalities. Enlarged perivascular spaces are commonly seen in chromosomal mosaicism,5,6 and it is probable that they also represent a developmental anomaly in the 6p25 deletion syndrome.
Cerebral white matter abnormalities in children are generally considered suggestive of an underlying metabolic defect. However, multifocal white matter lesions are most commonly seen in static, nonmetabolic encephalopathies2 with congenital CMV infection high in the differential diagnosis.2–4 Chromosomal abnormalities should also be considered. The 6p25 deletion syndrome should be specifically considered in patients presenting with a combination of psychomotor retardation, anterior eye chamber abnormalities, sensorineural deafness, dysmorphic features, and, on MR imaging, multifocal cerebral white matter lesions with enlarged perivascular spaces. Standard chromosome analysis is usually unrevealing, and the diagnosis should be confirmed by FISH analysis.
References
- Received July 9, 2005.
- Accepted after revision August 2, 2005.
- Copyright © American Society of Neuroradiology